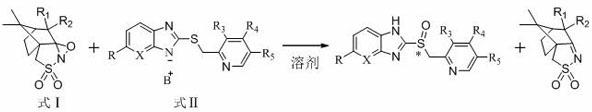
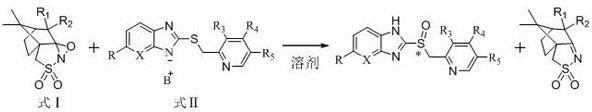
Preparation method of chiral sulphoxide proton pump inhibitor or pharmaceutically-acceptable salt thereof
A proton pump inhibitor and sulfoxide technology, which is applied in the field of preparation of chiral sulfoxide proton pump inhibitors or their pharmaceutically acceptable salts, can solve the problem of low stereoselectivity, low stereoselectivity of chiral sulfoxide proton pump inhibitors, Absolute configuration changes, application limitations, etc., to achieve the effects of specific stereoselectivity, low production cost, and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Example 1 Preparation of Chiral Sulfoxide Proton Pump Inhibitors
[0040] 1) Preparation of omeprazole sulfide diazabicyclic (DBU) complex
[0041] Dissolve 0.5 g of omeprazole sulfide in 6 mL of isopropanol, drop 0.23 mL of diazabicyclo (DBU) into the reaction under stirring conditions, and stir for 30 minutes at room temperature. Concentration under reduced pressure gave 0.72 g of a light yellow oily substance with a yield of 98%.
[0042] 1 H-NMR (400 MHz, d 6 -DMSO): δ = 8.14 (s, 1H), 7.12 (d, J = 8.4 Hz, 1H), 6.82 (d, J = 2.4 Hz, 1H), 6.45 (dd, J 1 = 2.4Hz, J 2 = 8.8 Hz, 1H), 5.39 (s, 1H), 4.57 (s, 2H), 3.71 (s, 3H), 3.70 (s, 3H), 3.21-3.17 (m, 4H), 3.09 (t, J = 5.6 Hz, 2H), 2.31-2.29 (m, 2H), 2.26 (s, 3H), 2.19 (s, 3H), 1.71-1.65 (m, 2H), 1.58-1.47 (m, 6H).
[0043] 2)( S )-Omeprazole preparation
[0044] The prepared 0.72 g omeprazole thioether diazabicyclic (DBU) complex was dissolved in 6 mL of isopropanol, and 0.35 g (1 R )-(-)-cam...
Embodiment 2
[0048] Example 2 Preparation of Chiral Sulfoxide Proton Pump Inhibitors
[0049] 1) ( S )-Omeprazole preparation
[0050] Dissolve 0.18 mL of DBU and 0.4 g of omeprazole sulfide in 5 mL of isopropanol, stir and react for 30 minutes at room temperature, then move to ice-bath conditions, add 0.28 g (1 R )-(-)-camphorsulfonazine, maintain ice-bath conditions, and stir the reaction. TLC detected that the reaction was complete. Suction filtration, the filter cake (the filter cake is camphorsulfonylimide compound, the recovery rate is 82%) was washed with isopropanol, after the filtrate was concentrated, about 2 mL of water was added, and the pH was adjusted to 7-10 with acetic acid solution, ethyl acetate The ester was extracted 3 times, and the ethyl acetate phase was dried and concentrated to obtain a foamy solid ( S )-omeprazole 0.35 g, yield: 83.3%.
[0051] HPLC determination ee: 99.18% (HPLC detection: chiral column AD, mobile phase: n-hexane / isopropanol = 60 mL / 4...
Embodiment 3
[0057] Example 3 The preparation of dexlansoprazole
[0058] Take 10g of 2-[3,5-dimethyl-4-(2,2,2-trifluoroethoxy)pyridine-2-methylenethio]-1H-benzimidazole, put it in 250ml In the bottle, add 120ml of isopropanol. Under the condition of stirring, add DBU (diazabicyclo) 3.9g, after stirring and reacting for 30 minutes, under the condition of ice bath, add D-camphorsulfonoxazine 6.4g in the reaction bottle, stir the reaction, TLC detects that the reaction is complete, Filter the reaction solution, wash the obtained solid with 20ml of isopropanol to obtain a light yellow filtrate, concentrate to dryness under reduced pressure, add 50ml of water, stir well, add 50% acetic acid solution dropwise to the aqueous solution, adjust pH = 8-8.5, acetic acid Ethyl 50ml×3 was extracted, the ethyl acetate layers were combined and dried over anhydrous sodium sulfate, filtered, and the ethyl acetate layer was concentrated under reduced pressure to obtain a light yellow oil. Add 20ml o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com