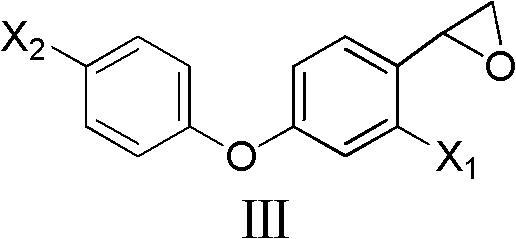
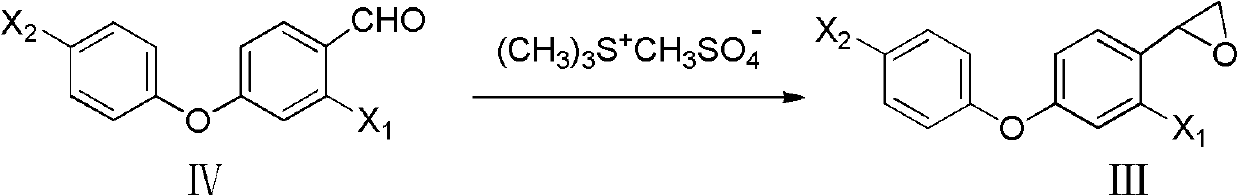2-(4-phenoxyphenyl)oxirane and preparation method and application thereof
A technology of phenoxyphenyl and ethylene oxide, which is applied in the application field of preparing difenoconazole fungicide, can solve the problems of low difenoconazole crude product content and high isomer content, and achieve Novel preparation process, high product purity and good quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
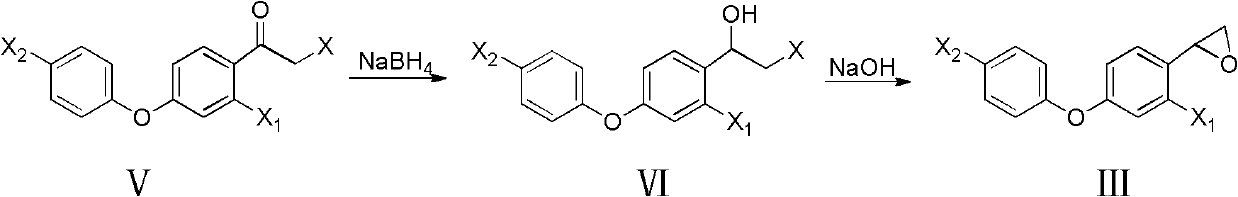
[0036] Preparation of 2-[2-chloro-4-(4-chlorophenoxy)-phenyl]oxirane
[0037]
[0038] 0.098mol 2-bromo-1-[2-chloro-4-(4-chlorophenoxy)phenyl]ethanone, 50mL tetrahydrofuran, add 0.049mol sodium borohydride under ice bath, TLC monitoring complete reaction; add 50mL hydrogen Sodium oxide solution, TLC monitors complete reaction; add dilute hydrochloric acid to neutrality, dichloromethane extracts, combine organic layers, dry over anhydrous sodium sulfate, spin dry to give light yellow liquid 2-[2-chloro-4-(4-chloro Phenoxy)-phenyl]oxirane, yield 86.0%. 1 H NMR (CDCl 3 , 400MHz) δ: 2.67 (dd, J=2.4Hz, J=5.6Hz, 1H, CH2), 3.18 (dd, J=3.2Hz, J=5.6Hz, 1H, CH2) 2 ), 4.16 (dd, J=2.4Hz, 3.2Hz, 1H, CH), 6.87 (dd, J=2.4Hz, J=8.4Hz, 1H, C 6 h 3 5-H), 6.95(d, J=8.8Hz, 2H, C 6 h 4 2,6-H), 6.98 (d, J=2.4Hz, 1H, C 6 h 3 3-H), 7.18(d, J=8.4Hz, 1H, C 6 h 3 6-H), 7.31(d, J=8.8Hz, C 6 h 4 3,5-H).
Embodiment 2
[0040] Preparation of 2-[2-chloro-4-(4-chlorophenoxy)-phenyl]oxirane
[0041] 0.098mol 2-chloro-1-[2-chloro-4-(4-chlorophenoxy)phenyl]ethanone, 50mL tetrahydrofuran, add 0.049mol sodium borohydride under ice-cooling, TLC monitoring complete reaction; add 50mL hydrogen Sodium oxide solution, TLC monitors complete reaction; add dilute hydrochloric acid to neutrality, extract with dichloromethane, combine organic layers, dry over anhydrous sodium sulfate, spin dry to give light yellow liquid 2-[2-chloro-4-(4-chloro Phenoxy)-phenyl]oxirane, yield 85.0%.
Embodiment 3
[0043] Preparation of 2-[2-chloro-4-(4-chlorophenoxy)-phenyl]oxirane
[0044] 1.0mol 2-chloro-4-(4-chlorophenoxy)benzaldehyde, 1.2mol sulfur ylide and 150ml toluene and dimethyl sulfide, add 3.0mol KOH solution under control at 10°C, after adding, continue to stir for a while , add dilute hydrochloric acid to neutralize to neutrality, then separate the liquids, and spin the solvent to obtain 2-[2-chloro-4-(4-chlorophenoxy)-phenyl]oxirane with a yield of 90.0%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com