Biodegradable temperature response hydrogel and preparation method thereof
A temperature-responsive, biodegradable technology, applied in the direction of medical preparations and pharmaceutical formulations of non-active ingredients, can solve the problems of non-biodegradable, limited application, low LCST, etc., achieve good biodegradability, broad application prospects, The effect of high mechanical strength
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
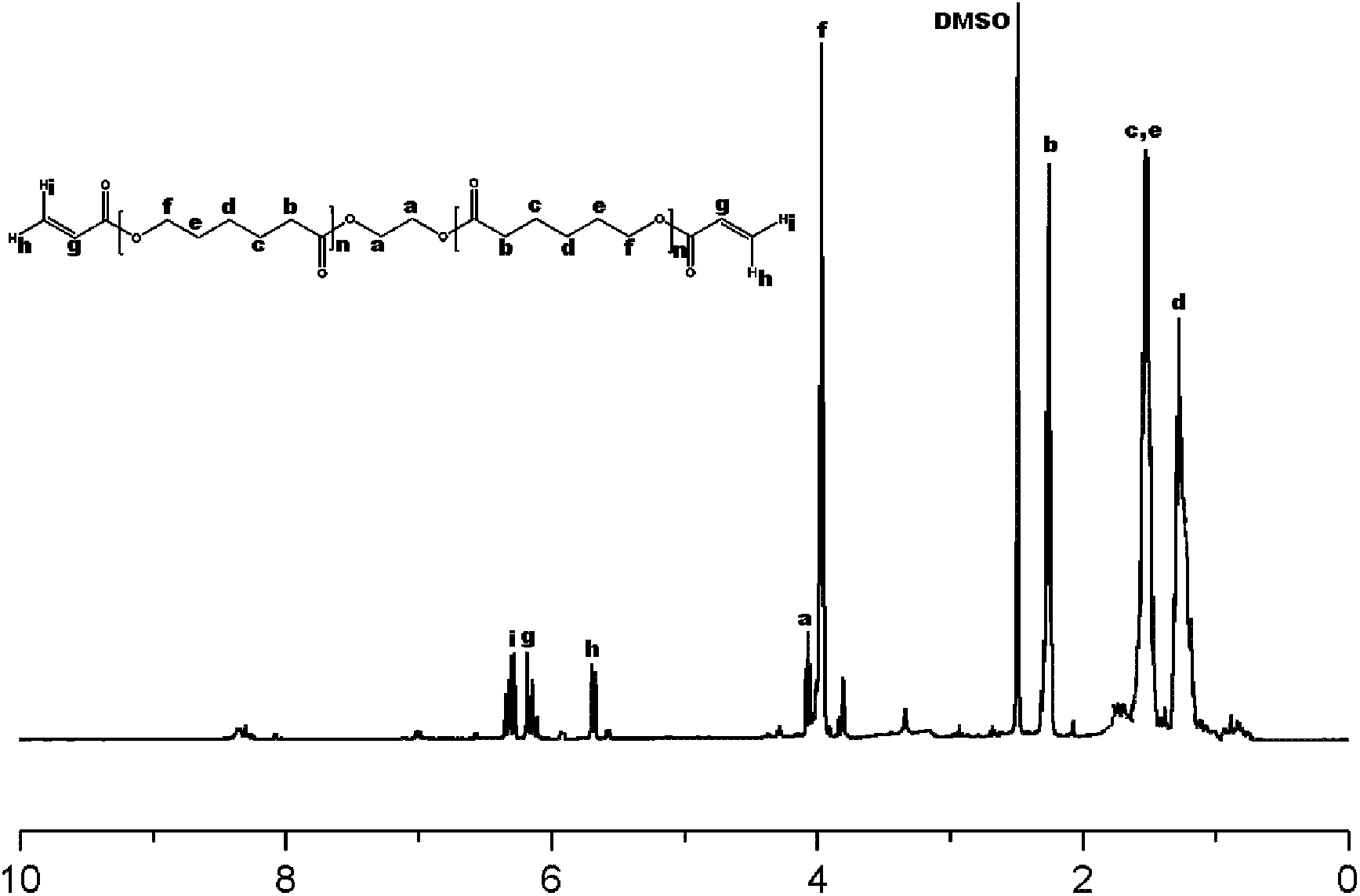
[0035] The preparation process of the used polycaprolactone macromolecular crosslinking agent (PCLDAC) of embodiment 3~6 is as follows: get 2.5g polycaprolactone glycol and dissolve in 20ml dichloromethane, add N, N-dicyclohexyl Carbodiimide 4.06g, 2-methylaminopyridine 0.1g, sealed and stirred at room temperature for 48h. After the reaction was completed, the insoluble matter was removed by suction filtration, and the filtrate was concentrated to 5 ml by rotary evaporation, poured into -20°C methanol for precipitation twice, and filtered. The obtained polycaprolactone macromolecular cross-linking agent was designated as PCLDAC. H of PCLDAC 1 NMR spectrum see figure 1 , the test result is: δ=1.225-1.294(m, 2H, -CH2-); δ=1.493-1.553(m, 4H, -CH2CH2-); δ=2.239-2.276(m, 2H, -CH2CO-); δ=3.950-3.983(t, 2H, -O-CH2-); δ=5.568-5.699, 6.278-6.324(m, 2H, =CH2); δ=6.145-6.186(t, 1H, -OCH=).
Embodiment 2
[0037] The preparation process of the polyethylene glycol monomethyl ether macromer (MPEGAC) used in embodiment 3~6 is as follows:
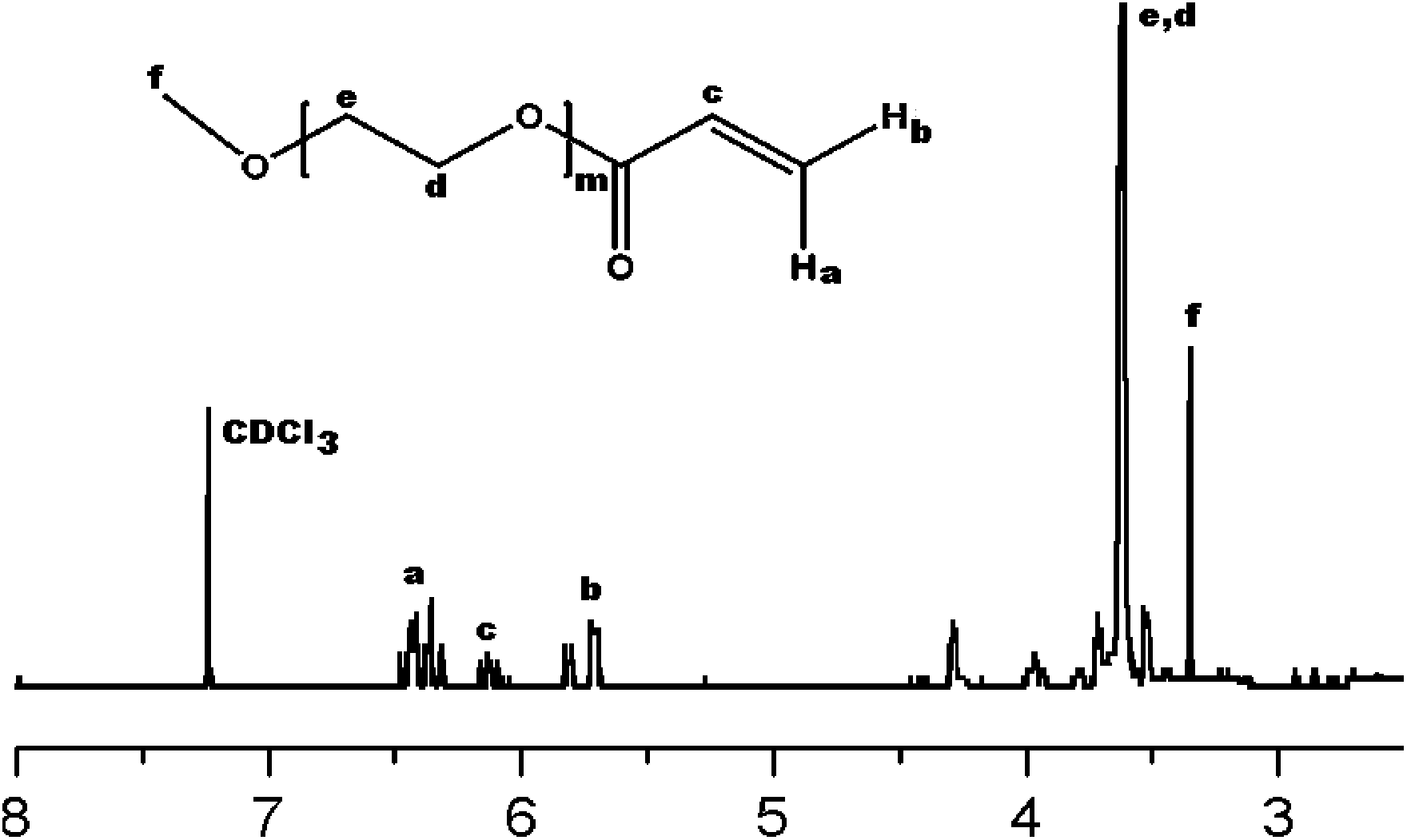
[0038] Take 2.5 g of polyethylene glycol monomethyl ether, dissolve it in 20 ml of dichloromethane, and then add 0.2 ml of triethylamine. Dissolve 0.25ml of acryloyl chloride in 5ml of dichloromethane to obtain a dichloromethane solution of acryloyl chloride. Add the dichloromethane solution of acryloyl chloride dropwise into the reactor under ice-bath conditions. Stir vigorously for 24 hours, remove the insoluble matter by suction filtration after the reaction is completed, concentrate the filtrate to 10ml by rotary evaporation at room temperature, pour it into petroleum ether and repeat the precipitation twice, filter to obtain the macromonomer of polyethylene glycol monomethyl ether, and the product is marked as MPEGAC . MPEGAC H 1 NMR spectrum see figure 2 , the test result is: δ=3.352 (s, 3H, -CH3); δ = 3.616-3.630 (d, 4H, -O-CH2CH2-O-);...
Embodiment 3
[0040] Weigh PCLDAC 0.0333g, MPEGAC 0.0667g, N-isopropylacrylamide 0.2g, azobisisobutyronitrile 3mg, dissolve in 1.5ml 1,4-dioxane, freeze, vacuumize, and fill with nitrogen And thawing, repeat 3 times, then seal the tube, react at 65°C for 24h, the LCST can be obtained at 32.7°C, the imbibition rate at 35°C is 0.95, the equilibrium state can be reached in 5h, and the biodegradable temperature of compressive strength is 54.5KPa Responsive hydrogels.
PUM
| Property | Measurement | Unit |
|---|---|---|
| strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com