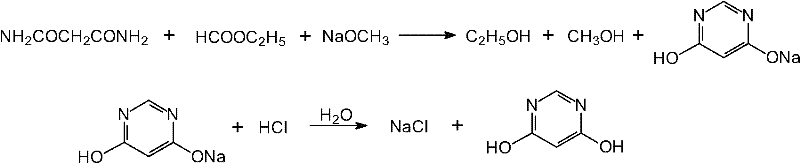
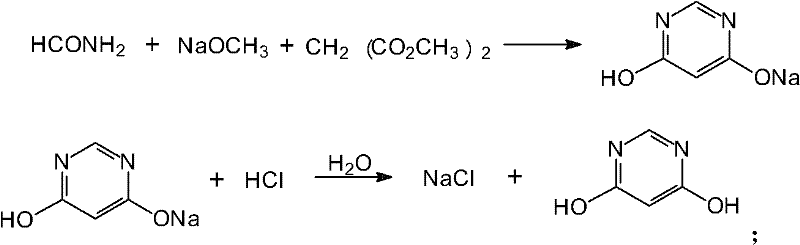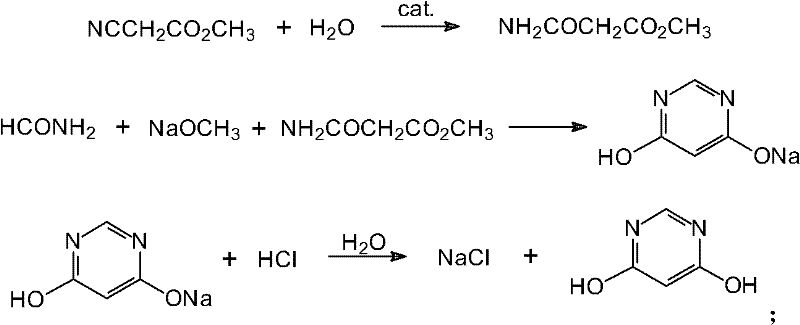Method for preparing 4,6-dihydroxy-pyrimidine from byproduct hydrocyanic acid of acrylonitrile
A technology of dihydroxypyrimidine and hydrocyanic acid, applied in 4 fields, to achieve the effect of less generation of three wastes, less environmental pollution and low product cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] In a 500ml reaction bottle, add 200ml of solvent toluene and 37g of methanol, stir and cool down to below -20°C, add 27g of hydrocyanic acid, start to pass in 42g of dry hydrogen chloride gas, control the temperature not to exceed 0°C for 8 hours, filter to obtain Aminomethyl ether hydrochloride.
[0024] For the iminomethyl methyl ether hydrochloride obtained in the previous step, add 93g of ammonium acetate and dissolve it in 200ml of anhydrous methanol in portions below -5°C, stir vigorously for 1 hour, heat up to room temperature and react for 2 hours, and heat up to The reaction was continued at 40°C for 1 hour, and white precipitated ammonium chloride gradually precipitated, cooled down, and filtered.
[0025] The filtrate is heated to reflux, and at the same time, dry ammonia gas is introduced at a certain speed for 2 to 2.5 hours, and about 85g of ammonia gas is introduced, and a white solid is gradually precipitated in the reaction bottle. After cooling down, f...
Embodiment 2
[0028] In a 500ml reaction bottle, add 200ml of solvent toluene and 46g of ethanol, stir and cool down to below -15°C, add 27g of hydrocyanic acid, start to pass in 40g of dry hydrogen chloride gas, control the temperature not to exceed -5°C for 6 hours, filter, and get Iminomethyl ether hydrochloride.
[0029] For the iminomethyl ethyl ether hydrochloride obtained in the previous step, add 85g of ammonium acetate in portions below -5°C and dissolve it in 200ml of absolute ethanol solution, stir vigorously for 1 hour, heat up to room temperature and react for 1 hour, then heat up to 45°C The reaction was continued for 1 hour, and white precipitated ammonium chloride was gradually precipitated, the temperature was lowered, and the mixture was filtered.
[0030] The filtrate is heated to reflux, and at the same time, dry ammonia gas is introduced at a certain speed for 1.5 to 2 hours, and about 35g of ammonia gas is introduced, and white solids are gradually precipitated in the ...
Embodiment 3
[0033] In a 500ml reaction bottle, add 200ml of solvent toluene and 46g of ethanol, stir and cool down to below -10°C, add 27g of hydrocyanic acid, start to pass in 40g of dry hydrogen chloride gas, control the temperature not to exceed 0°C for 6 hours, filter to obtain Aminomethyl ether hydrochloride.
[0034] For the iminomethyl ethyl ether hydrochloride obtained in the previous step, add 78g of ammonium acetate in portions below 0°C and dissolve it in 200ml of absolute ethanol solution, stir vigorously for 1 hour, heat up to room temperature and react for 1 hour, then raise the temperature to 45°C to continue After reacting for 1 hour, white precipitated ammonium chloride gradually precipitated, cooled down, and filtered.
[0035] The filtrate is heated to reflux, and at the same time, dry ammonia gas is introduced at a certain speed for 1.5 to 2 hours, and about 35g of ammonia gas is introduced, and white solids are gradually precipitated in the reaction bottle. After cool...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com