Method for removing anion pollutants from water
An anionic pollutant and water removal technology, applied in the field of purification treatment, can solve the problems of high economic cost, high economic cost and operating cost, non-recyclable use, etc., to achieve increased adsorption surface area, good application prospects, and mild preparation conditions. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
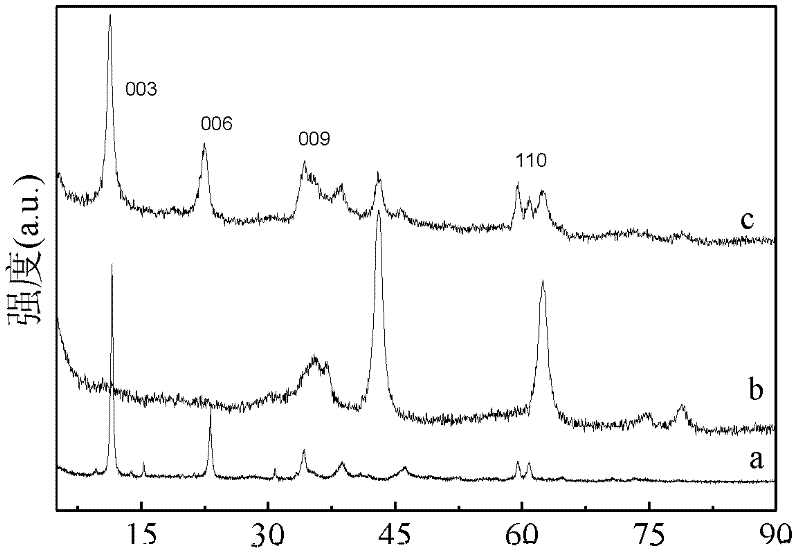
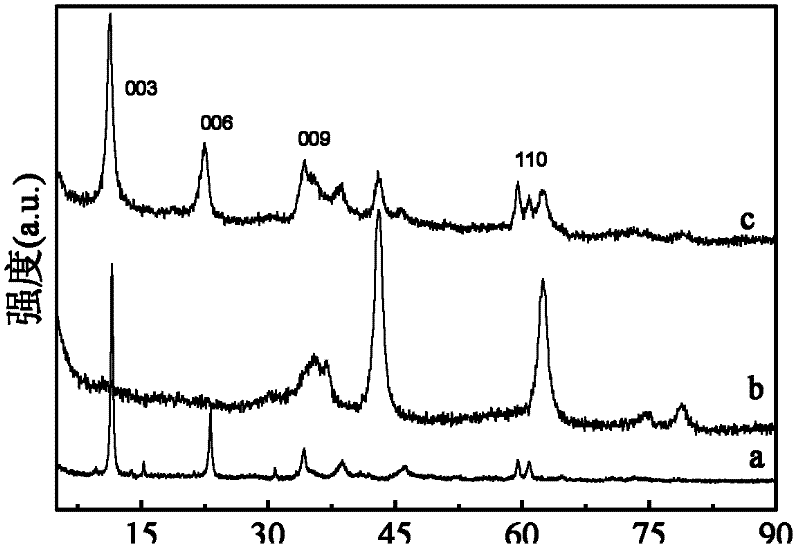
Image
Examples
Embodiment 1
[0044] (1) Weigh 192.3075g of magnesium nitrate hexahydrate and 101.005g of ferric nitrate nonahydrate, pour them into a 500mL conical flask, mix well and then add 150mL of deionized water to dissolve. If there are fine particles that cannot be dissolved, ultrasonication can be used for 3 to 5 minutes After stirring on an electromagnetic stirrer, the mixed solution that is completely dissolved after half an hour is called a. Weigh 80.02g of anhydrous sodium hydroxide and 52.995g of anhydrous sodium carbonate respectively, pour them into another 500mL conical flask, and quickly add 150mL of deionized water to dissolve them. If there are fine particles that cannot be dissolved, use ultrasonic After 3 to 5 minutes, electromagnetically stir evenly to obtain a completely dissolved solution b.
[0045] (2) Clean the two 150mL constant pressure funnels, dry them, and check their tightness. When ready, transfer most of the solutions a and b to the two constant pressure funnels, and re...
Embodiment 2
[0056] (1) Repeat steps (1) to (5) of Example 1.
[0057] (2) Deionized water is respectively configured with ClO 4 - Concentration 2000μg / L, BrO 3 - Concentration 2000 - μg / L, NO 3 - Concentration 5000μg / L, Cl - Concentration 5000μg / L, H 2 PO 4 - Concentration 5000μg / L, SO 4 2- Concentration 5000μg / L, CO 3 2- A solution with a concentration of 5000 μg / L. Use graduated cylinders to pipette 200mL of the mixed solution into 250mL ground-necked flasks.
[0058] (3) Put 0.5 g of magnesium-iron layered double metal oxide with a particle size of 140 mesh into the above-mentioned deionized water containing anion pollutants (the weight ratio of adsorbent to water is 1:400), and put After shaking for 12 hours at a rotating speed of 200rpm, 5mL of the adsorption liquid was filtered through a 0.45μm filter membrane, and the adsorption rate of the adsorbent for each anion pollutant was determined by ion chromatography.
[0059] After 12h of adsorption was determined by ion...
Embodiment 3
[0061] (1) Repeat steps (1) to (5) of Example 1.
[0062] (2) configure ClO 4 -Each 200mL solution with an initial concentration of 2000μg / L and pH values of 2, 4, 6, 8, 10, and 12 was added with 0.5g of adsorbent (the weight ratio of adsorbent to water was 1:400). Put it into a shaking table at a speed of 200rpm, shake for 12 hours, take 5mL of the adsorption solution and filter it through a 0.45μm filter membrane, and measure the removal efficiency of the adsorbent for perchlorate by ion chromatography.
[0063] (3) Experiments showed that the removal rates of perchlorate with pH values of 2, 4, 6, 8, 10, and 12 by the adsorbent were 45%, 88.51%, 90.40%, 89.92%, 85.92, and 53.76%, respectively.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com