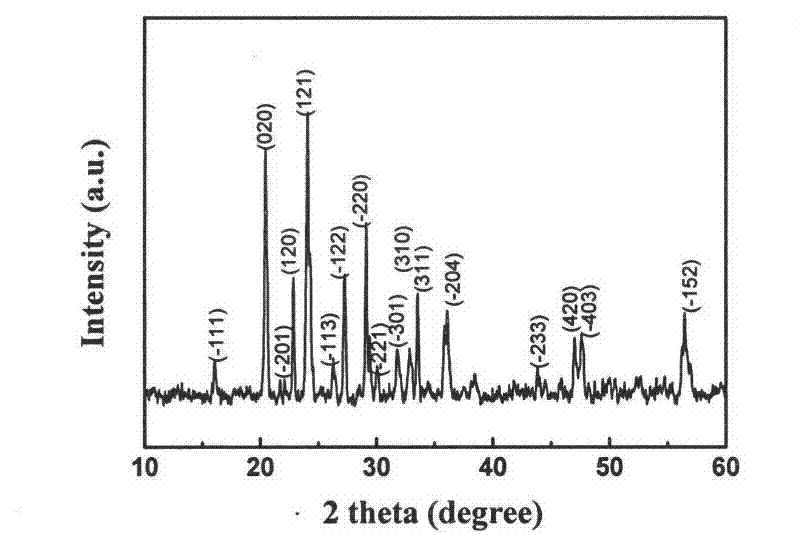
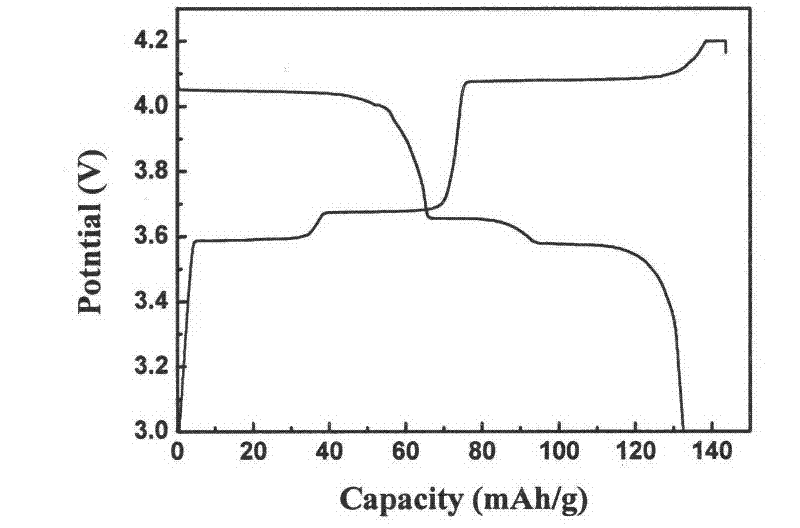
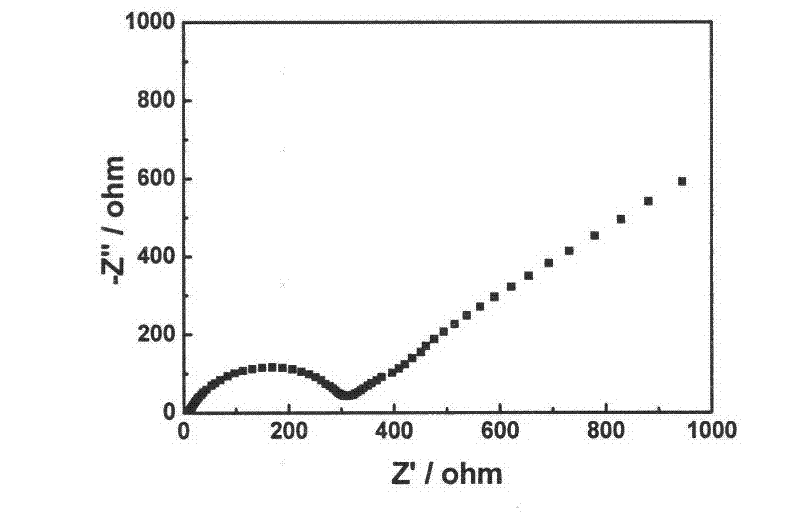
Rheological phase method for preparing sodium-doped positive pole material lithium vanadium phosphate of lithium-ion battery
A technology of lithium ion battery and cathode material, applied in battery electrodes, circuits, electrical components, etc., to achieve high discharge voltage, high charge and discharge capacity, and reduce pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] (1) Take 3.0313g vanadium pentoxide (V 2 o 5 ) in the beaker, adding mass percentage concentration is 10% hydrogen peroxide 30ml, stirs with glass rod, forms vanadium pentoxide hydrogel (V 2 o 5 ·nH 2 O);
[0019] (2) Add 6.493g of diammonium hydrogen phosphate 6.493g, 2.063g of lithium hydroxide monohydrate 2.063g, 0.1100g of sodium salt and 0.1000g of high molecular weight PEG35000 to the product obtained in step (1), and ultrasonically vibrate for 5 minutes;
[0020] (3) Use a 5ml pipette to measure 1.05ml of analytically pure small molecular weight PEG200 into a beaker vibrated by ultrasonic waves, transfer it to a round bottom flask, and evaporate it to dryness on a rotary evaporator at 40°C for 1 hour, and then dry it in a vacuum. Dry in the oven at 100°C for 4 hours; take it out, grind it into powder with an agate mortar, put the powder in a porcelain boat, sinter in a vacuum sintering furnace at 650°C under the protection of high-purity argon for 10 hours, a...
Embodiment 2
[0024] (1) Take 3.0313g vanadium pentoxide (V 2 o 5 ) in the beaker, adding mass percentage concentration is 30% hydrogen peroxide 30ml, stirs with glass rod, forms vanadium pentoxide hydrogel (V 2 o 5 ·nH 2 O);
[0025] (2) Add 6.493g of diammonium hydrogen phosphate 6.493g, 2.063g of lithium hydroxide monohydrate 2.063g, 0.1100g of sodium salt and 0.1g of high molecular weight PEG20000 to the product obtained in step (1), and ultrasonically vibrate for 5 minutes;
[0026] (3) Use a 5ml pipette to measure 2.05ml of analytically pure small molecular weight PEG600 into a beaker vibrated by ultrasonic waves, transfer it to a round bottom flask, and evaporate it to dryness on a rotary evaporator at 50°C for 0.5 hours, and then dry it in a vacuum. Dry in the box at 100°C for 4 hours; take it out, grind it into powder with an agate mortar, put the powder in a porcelain boat, sinter in a vacuum sintering furnace at 850°C under the protection of high-purity argon for 20 hours, an...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Charge and discharge rate | aaaaa | aaaaa |
| Charge and discharge efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com