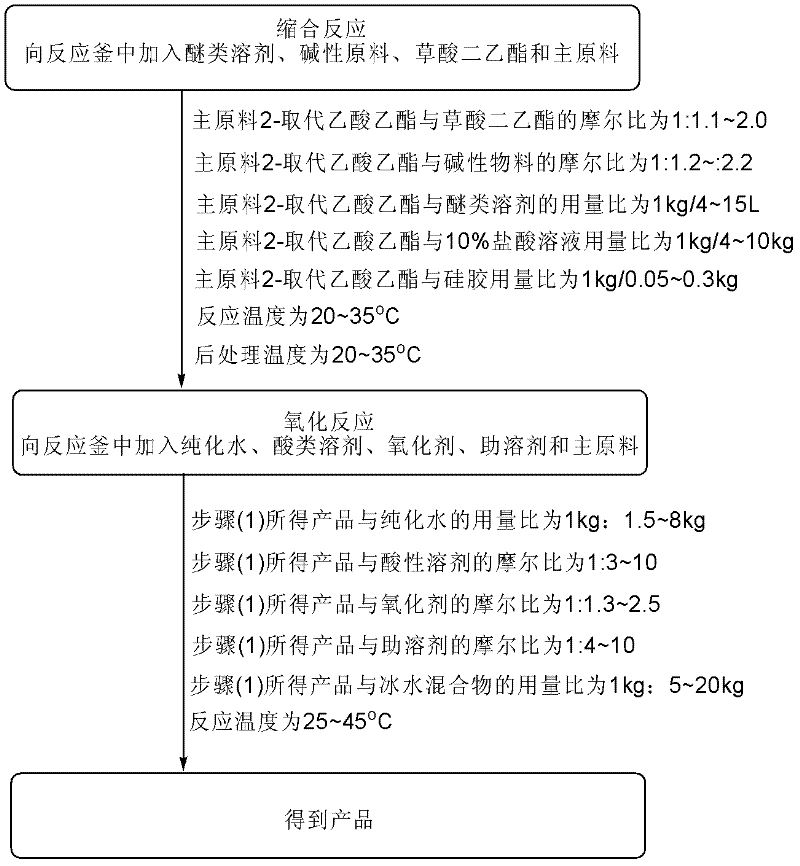
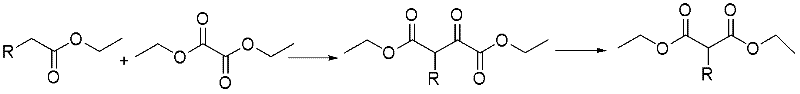
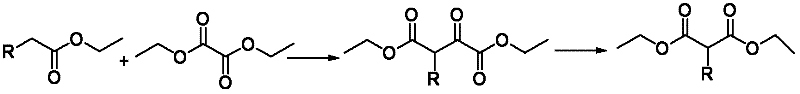
Method for preparing alpha-replacing malonic acid diacetoxyiodo derivative
A technology of diethyl malonate and diethyl oxosuccinate, which is applied in the field of preparing diethyl malonate derivatives, can solve the problems of complex process, large environmental pollution, harsh reaction conditions, etc. The effect of stable conditions, safe process and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Embodiment 1: Preparation of diethyl 2-fluoromalonate
[0026](1) Condensation: In a 1000L reactor, add 614.0kg tetrahydrofuran (1kg / 6L) at one time, add 156.3kg sodium tert-butoxide (1.5eq) and 136.5kg diethyl oxalate (1.3eq) in batches, and control the temperature 25±2°C, add 115kg of main raw material ethyl fluoroacetate (1.0eq) dropwise to the system, and keep it warm for reaction. 6kg) to adjust pH=2, then extract and wash, in addition, add 17.3kg silica gel (1g / 0.15g) to remove tar, finally dry, press filter, and concentrate to obtain the product 2-fluoro-3-oxosuccinic acid Diethyl ester 217.2kg, yield 72.9%, external standard (Wt%): 75%, liquid chromatography purity (HPLC): 91.2%.
[0027] (2) Oxidation: Add 240.0kg of purified water (1kg / 2kg) to the 1000L reaction kettle, add 286.0kg of concentrated sulfuric acid (5eq) and 283.1kg of potassium persulfate (1.8eq) to the kettle in batches, and then add 134.0 kg co-solvent absolute ethanol (5eq), strictly contr...
Embodiment 2
[0028] Embodiment 2: prepare diethyl methylmalonate
[0029] (1) Condensation: In a 500L reactor, add 555.3kg methyl tert-butyl ether (1kg / 15L) at one time, add 57.5kg potassium tert-butoxide (1.2eq) and 124.7kg diethyl oxalate (2.0 eq), temperature control 20±2°C, add 50kg main raw material ethyl propionate (1.0eq) dropwise to the system, heat preservation reaction, after the reaction is complete, carry out post-treatment with temperature control 20±2°C, use 500kg10% hydrochloric acid solution (1kg / 10kg) to adjust the pH=1, then extract and wash, in addition, add 15.0kg silica gel (1kg / 0.3kg) to remove tar, finally dry, press filter, and concentrate to obtain the product 2-methyl-3-oxo Diethyl succinate 80.5kg, yield 65.3%, external standard (Wt%): 70%, liquid chromatography purity (HPLC): 91%.
[0030] (2) Oxidation: Add 400kg of purified water (1kg / 8kg) to the 500L reactor, add 90.3kg of concentrated hydrochloric acid (10eq) and 76.5kg of sodium persulfate (1.3eq) to the...
Embodiment 3
[0031] Embodiment 3: Preparation of 2-hydroxydiethyl malonate
[0032] (1) Condensation: In a 500L reactor, add 206.5kg 2-methyltetrahydrofuran (1kg / 4L) at one time, add 86.3kg sodium ethoxide (2.2eq) and 92.7kg diethyl oxalate (1.1eq) in batches, Control the temperature at 28±2°C, add 60kg of main raw material 2-hydroxyacetate ethyl ester (1.0eq) dropwise to the system, and keep it warm for reaction. 10% hydrochloric acid solution (1kg / 4kg) to adjust the pH=3, then extract and wash, add 3.0kg silica gel (1g / 0.05g) to remove tar, finally dry, press filter and concentrate to obtain the product 2-hydroxyl -Diethyl 3-oxosuccinate 110.7kg, yield 68.0%, external standard (Wt%): 72%, liquid chromatography purity (HPLC): 90.8%.
[0033] (2) Oxidation: Add 82.5kg of purified water (1kg / 1.5kg) to the 500L reaction kettle, add 30.0kg of concentrated hydrochloric acid (3eq) and 153.6kg of ammonium persulfate (2.5eq) to the kettle in batches, and then add 156.5kg cosolvent acetone (10...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com