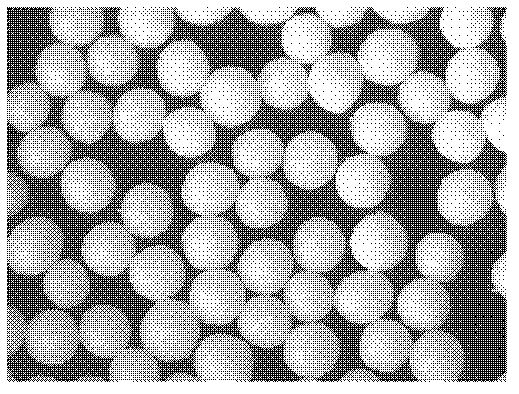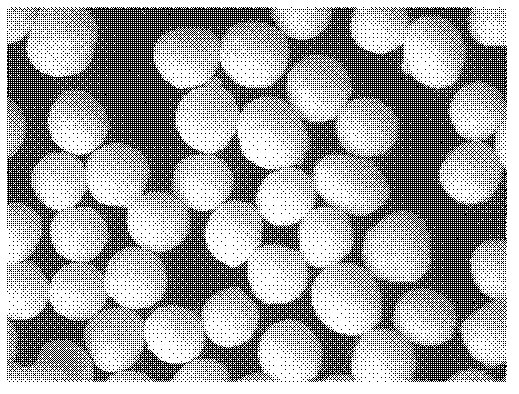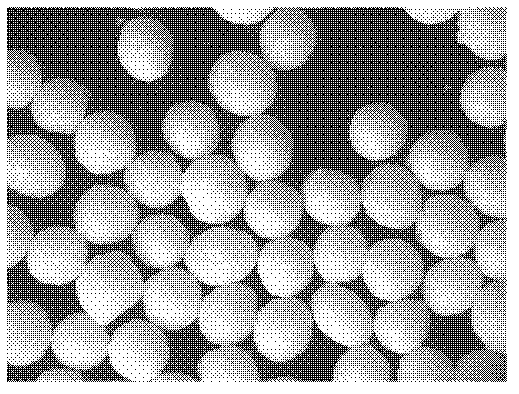Metoprolol tartrate sustained release pellet and preparation method thereof
A technology of metoprolol tartrate and sustained-release pellets, which can be used in pharmaceutical formulations, medical preparations containing active ingredients, and block transportation, etc., which can solve problems such as danger, difficulty in recycling, and unsafety, and achieve production costs Low, uniform particle size distribution, high true sphericity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066] Ball core formula: parts by weight
[0067] Metoprolol tartrate 100 parts
[0068] 95% ethanol 50 parts
[0069] Sustained-release coat formula (taking metoprolol tartrate core as 100 parts by weight):
[0070] Hypromellose 5 parts
[0071] 10% Surelease 250 parts of water dispersion
[0072] Note: 10% Surelease The aqueous dispersion is ethyl cellulose aqueous dispersion.
[0073] Preparation process: prepare three batches of ball cores according to the "Example Description" and the above formula, the quality evaluation results are shown in Table 3 below, and the properties of the ball cores are shown in Table 3. Picture 1-1 , 1-2 , 1-3.
[0074] Table 3: Ball core quality evaluation
[0075] Evaluation index / batch Batch 1 Batch 2 Batch 3 aspect ratio (E) 1.07 1.1 1.08 Comprehensive form factor (eR) 0.97 0.94 0.98 Friability (Fl) 0.007 0.007 0.008 Yield (Yd) 0.85 0.88...
Embodiment 2
[0077] Ball core formula: parts by weight
[0078] Metoprolol tartrate 100 parts
[0079] 75% ethanol 50 parts
[0080] Isolation gown formula (taking metoprolol tartrate core as 100 parts by weight):
[0081] 5% hydroxypropyl methylcellulose aqueous solution 80 parts
[0082] Sustained-release coat formula (based on 100 parts by weight of the metoprolol tartrate ball core of the pack isolation coat):
[0083] 10% Surelease Water dispersion 200 parts
[0084] Note: 10% Surelease The aqueous dispersion is ethyl cellulose aqueous dispersion.
[0085] Preparation process: Prepare three batches of ball cores according to the "Example Description" and the above formula, the quality evaluation results are shown in Table 4 below, and the properties of the ball cores are shown in Table 4. diagram 2-1 , 2-2 , 2-3.
[0086] Table 4: Ball core quality evaluation
[0087] Evaluation index / batch Batch 1 Batch 2 Batch 3 aspect ratio (E) 1.04 1.07 ...
Embodiment 3
[0089] Ball core formula: parts by weight
[0090] Metoprolol tartrate 100 parts
[0091] 70% ethanol 40 parts
[0092] Sustained-release coat formula (taking metoprolol tartrate core as 100 parts by weight):
[0093] 10% Eudragit NE30D water dispersion 300 parts
[0094] Note: Utage NE30D aqueous dispersion is the aqueous dispersion of ethyl acrylate-methyl methacrylate copolymer (2:1).
[0095] Preparation process: prepare three batches of ball cores according to the "Example Description" and the above formula, the quality evaluation results are shown in Table 5 below, and the properties of the ball cores are shown in Table 5. Figure 3-1 , 3-2 , 3-3.
[0096] Table 5: Ball core quality evaluation
[0097] Evaluation index / batch Batch 1 Batch 2 Batch 3 aspect ratio (E) 1.07 1.1 1.06 Comprehensive form factor (eR) 0.97 0.95 0.98 Friability (Fl) 0.008 0.01 0.007 Yield (Yd) 0.84 ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle size (mesh) | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com