Carbinoxamine sustained-release orally disintegrating tablets and preparation method thereof
A technology of orally disintegrating tablets and carbinoxamine, which is applied in the field of slow-release orally disintegrating tablets and its preparation, achieves the effects of simple preparation process, high true sphericity, and extremely easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
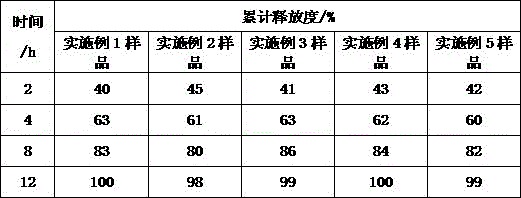
Examples
Embodiment 1
[0023] Prescription composition:
[0024] Sustained-release pellet composition:
[0025] Carbinoxamine 100g
[0026] Lactose 40g
[0027] Hypromellose 40g
[0028] Acrylic resin 10g
[0029] Polyethylene glycol 4g
[0031] Methylcellulose 4g
[0032] 200g total.
[0033] Sustained-release orally disintegrating tablet composition:
[0034] Carbinoxamine Sustained Release Pellets 150g
[0035] Croscarmellose Sodium 10g
[0036] Mannitol 30g
[0037] Aspartame 4g
[0039] Menthol 1g
[0041] 200g total.
[0042] Preparation Process:
[0043] Preparation of drug-containing pellets: Weigh the raw material of carbinoxamine, add lactose and hypromellose, and mix well; add an appropriate amount of water to the mixed material to make a soft material; put the soft material into an extrusion spheronizer Prepare drug-containing pellets; bake the prepared pellets at 50°C for 3-6 hours to obtain ...
Embodiment 2
[0047] Prescription composition:
[0048] Sustained-release pellet composition:
[0049] Carbinoxamine 100g
[0050] Microcrystalline Cellulose 35g
[0051] Hypromellose 45g
[0052] Ethyl cellulose 10g
[0053] Polyethylene glycol 4g
[0055] Methylcellulose 4g
[0056] 200g total.
[0057] Sustained-release orally disintegrating tablet composition:
[0058] Carbinoxamine Sustained Release Pellets 150g
[0059] Croscarmellose Sodium 5g
[0060] Microcrystalline Cellulose 35g
[0061] Aspartame 5g
[0062] Talc powder 3g
[0064] 200g total.
[0065] Preparation Process:
[0066] Preparation of drug-containing pellets: Weigh the raw material drug of carbinoxamine, add microcrystalline cellulose and hypromellose, and mix well; add an appropriate amount of water to the mixed material to make a soft material; put the soft material into extrusion spheronization Prepare drug-containing pellets in a granulator; dr...
Embodiment 3
[0070] Prescription composition:
[0071] Sustained-release pellet composition:
[0072] Carbinoxamine 100g
[0073] Mannitol 35g
[0074] Hypromellose 45g
[0075] Acrylic resin 10g
[0076] Triethyl citrate 4g
[0077] Talc powder 2g
[0078] Gelatin 4g
[0079] 200g total.
[0080] Sustained-release orally disintegrating tablet composition:
[0081] Carbinoxamine Sustained Release Pellets 150g
[0082] Cross-linked polyvinylpyrrolidone 10g
[0083] Mannitol 30g
[0084] Aspartame 5g
[0085] Talc powder 3g
[0087] 200g total.
[0088] Preparation Process:
[0089] Preparation of drug-containing pellets: Weigh the raw material of carbinoxamine, add mannitol and hypromellose, and mix well; add an appropriate amount of water to the mixed material to make a soft material; put the soft material into extrusion, spheronize and granulate Prepare drug-containing pellets in the machine; dry the prepared pellets at 50°C for 3-6 hours to ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com