Quality test method for hawthorn leaf medicine, hawthorn leaf extractives or products
A technology of hawthorn leaf extract and quality detection method, which is applied in the directions of drug combination, pharmaceutical formula, medical preparation containing active ingredients, etc. problems, to achieve moderate pH, high recovery and accuracy, and extended service life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
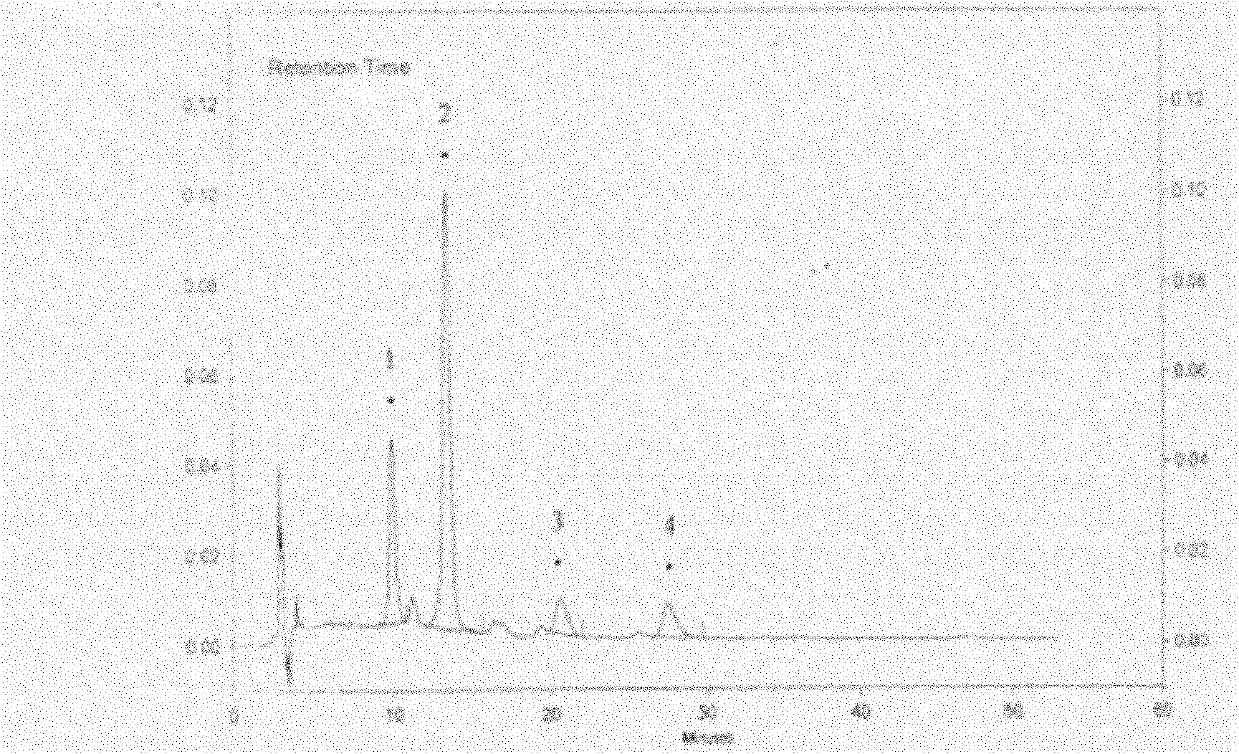
[0250] Embodiment 1: Determination of hyperin in hawthorn leaf medical material
[0251]a. Preparation of reference substance solution: take about 10.55 mg of hyperin reference substance, weigh it accurately, add 45% ethanol to dissolve and dilute, and make a solution containing 21.10 μg per 1 ml;
[0252] b. Preparation of the test solution: take 1g of hawthorn leaf medicinal material fine powder, accurately weighed as 1.002g, put it in a Soxhlet extractor, add chloroform and heat under reflux to extract until the extract is colorless, discard the chloroform , the medicinal dregs are evaporated to remove chloroform, add methanol and continue to extract until colorless, the extract is evaporated to dryness, the residue is dissolved in 45% ethanol, transferred to a 50ml measuring bottle, added 45% ethanol to the mark, shaken up, and used as Test sample stock solution: get the test sample stock solution, filter, accurately measure 5ml of the continued filtrate, put it in a 25ml ...
Embodiment 2
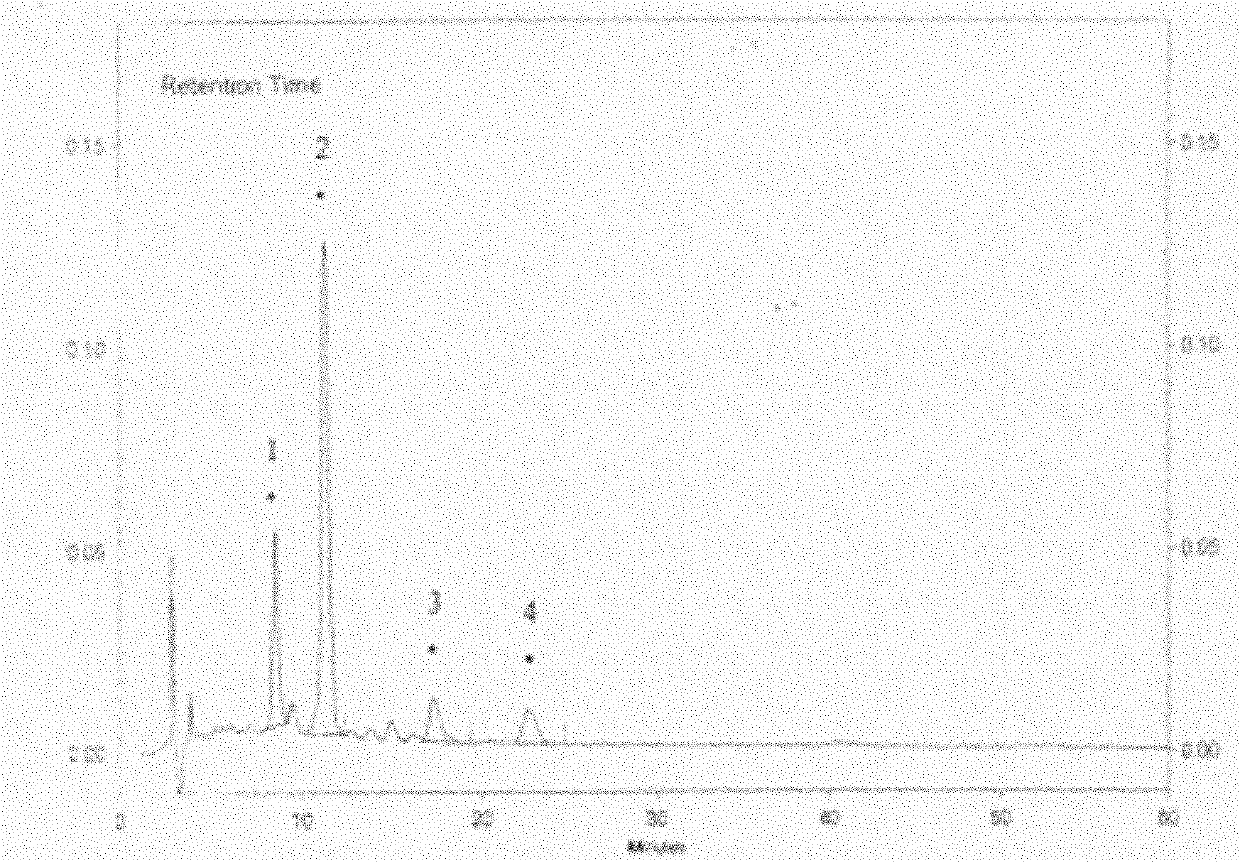
[0259] Example 2: Determination of active ingredients in hawthorn leaf extract
[0260] Hawthorn leaf extract is recorded in "Chinese Pharmacopoeia" 2010 edition under Yixindone Tablets. Its preparation method: take 1 part by weight of the leaves of Rosaceae plant Shanlihong or Hawthorn, grind it into coarse powder, and use 6 parts by volume of ethanol for Solvent, soak for 48 hours, carry out percolation, collect the filtrate, recover ethanol under reduced pressure to a concentrated solution with a relative density of about 1.04 (60°C), add an equal amount of water to dilute, and use 1 / 6 volume parts of petroleum ether (60~ 90°C) to remove the pigment, separate the water layer, shake and extract with 0.7 parts by volume of ethyl acetate, recover the ethyl acetate under reduced pressure from the extract and concentrate to dryness to obtain the product.
[0261] a. Preparation of reference substance solution:
[0262] Hyperin reference substance solution: take about 10.33mg of...
Embodiment 3
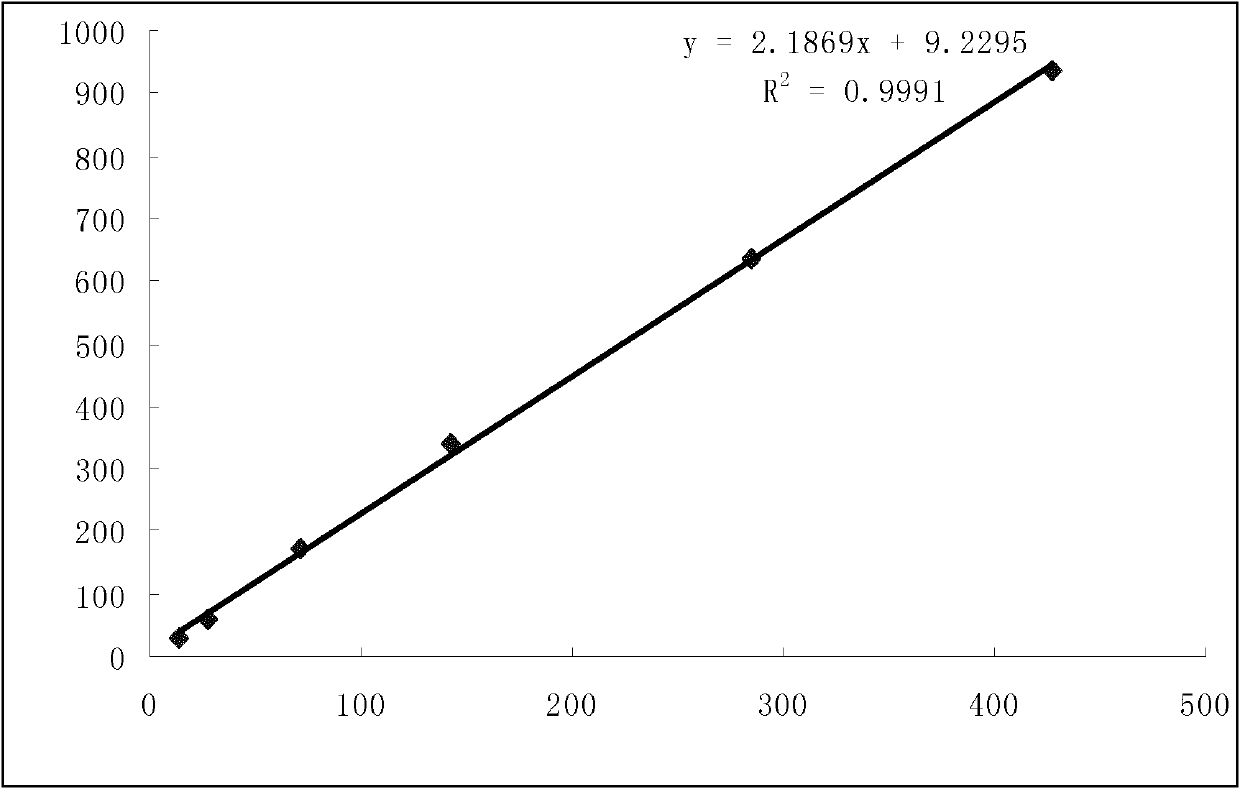
[0271] Example 3: Determination of hyperin in total glycosides of gypenosides and total flavonoids of hawthorn leaves composition (3)
[0272] The total flavonoid composition of gypenosides and hawthorn leaves (3) preparation method: take 20 parts by weight of gypenosides and 40 parts by weight of total flavonoids of hawthorn leaves, add 120 parts by weight of starch, mix evenly, granulate with starch slurry, dry, pack into Capsules, made into 1000 capsules, ready to use.
[0273] The total glycosides of Gypenopephylloides are recorded on page 31 of the fourth volume of the State Food and Drug Administration's New Drug Standards.
[0274] a. Preparation of reference substance solution: take 10.19 mg of hyperin reference substance, accurately weigh it, add 50% ethanol to dissolve and dilute, and make a solution containing 10.19 μg per 1 ml;
[0275] b. Preparation of the test solution: Accurately weigh 0.1311g of the content of the total glycosides and hawthorn leaf flavonoids...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com