Adriamycin nano-particles and preparation method thereof
A doxorubicin and nanoparticle technology, which is used in pharmaceutical formulations, medical preparations with non-active ingredients, and medical preparations containing active ingredients, etc., to achieve good stability, good biocompatibility, and good biodegradability. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0061] The invention provides a method for preparing doxorubicin nanoparticles, comprising the following steps:
[0062] Doxorubicin is electrostatically compounded with a block copolymer having a structure of formula (I) or formula (II) in an aqueous medium to obtain doxorubicin nanoparticles.
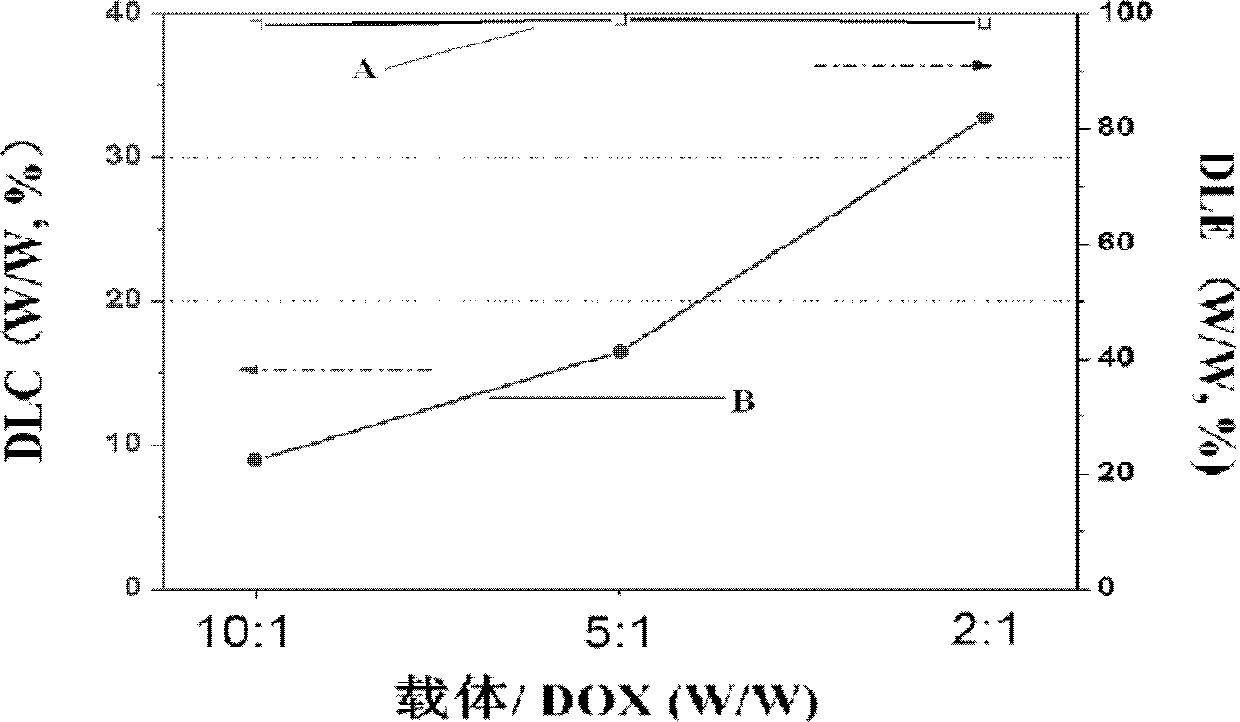
[0063] According to the preparation method of doxorubicin nanoparticles of the present invention, the obtained doxorubicin nanoparticles exist in the aqueous medium in the form of micelles, and the fluid radius of the micelles is preferably 10 nm to 2000 nm, more preferably 10 nm to 2000 nm. 600nm.
[0064] Since the doxorubicin nanoparticles that exist in the form of micelles are not conducive to preservation, it is preferred to obtain the freeze-dried powder of doxorubicin nanoparticles through post-processing, and the post-processing preferably includes the following steps:
[0065] After obtaining the doxorubicin nanoparticle micelles, dialyze for 24h-72h, change the water for 6-...
Embodiment 1
[0083] The block copolymer having the structure of formula (III-a), m=45 and x=10 is denoted as mPEG 45 -b-PPLG 10 .
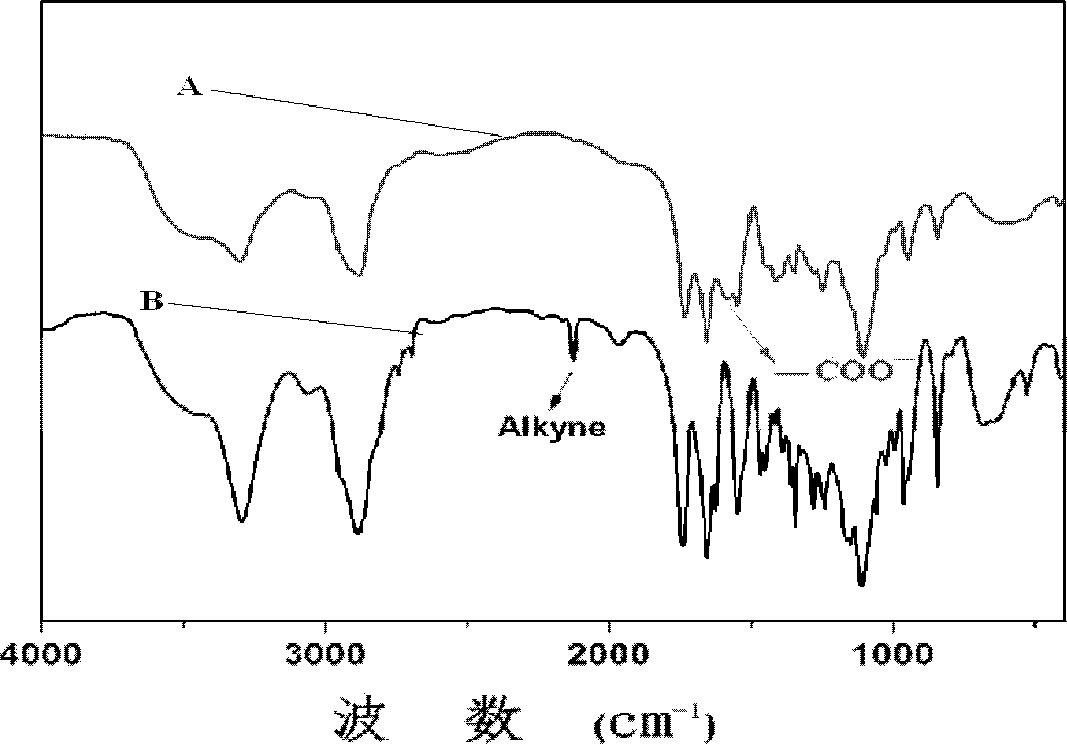
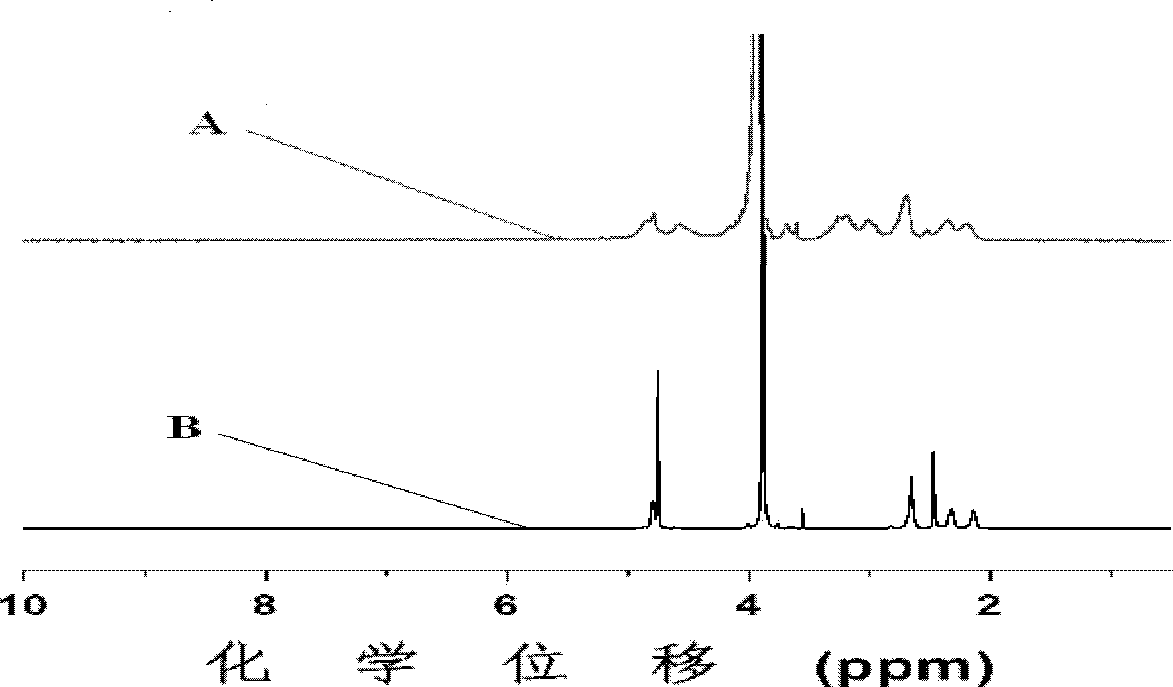
[0084] Add 0.2009g mPEG to the dry reaction vial 45 -b-PPLG 10 , after stirring and dissolving with 5mL N,N-dimethylformamide, after passing nitrogen for 0.5 hours, add 13.9mg benzoin dimethyl ether and 0.1636g mercaptosuccinic acid at room temperature under the condition of nitrogen protection, and react with 365nm ultraviolet light for 2h , to obtain the product; the product was dialyzed for 72 hours, the water was changed 12 times, and freeze-dried to obtain a block copolymer freeze-dried powder with a structure of formula (I-a).
[0085] The nuclear magnetic resonance test was carried out on the block copolymer, and the grafting rate was calculated to be 40.1%, and the reaction conversion rate was 68.5%.
Embodiment 2
[0087] The block copolymer having the structure of formula (III-a), m=45 and x=10 is denoted as mPEG 45 -b-PPLG 10 .
[0088] Add 0.2003g mPEG to the dry reaction vial 45 -b-PPLG 10 , after stirring and dissolving with 5mL N,N-dimethylformamide, after passing nitrogen for 0.5 hours, add 14.0mg benzoin dimethyl ether and 0.6543g mercaptosuccinic acid at room temperature under the condition of nitrogen protection, and react with 365nm ultraviolet light for 2h , to obtain the product; the product was dialyzed for 72 hours, the water was changed 12 times, and freeze-dried to obtain a block copolymer freeze-dried powder with a structure of formula (I-a).
[0089] The nuclear magnetic resonance test was carried out on the block copolymer, and the grafting rate was calculated to be 61.8%, and the reaction conversion rate was 30.2%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| radius | aaaaa | aaaaa |
| degree of grafting | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com