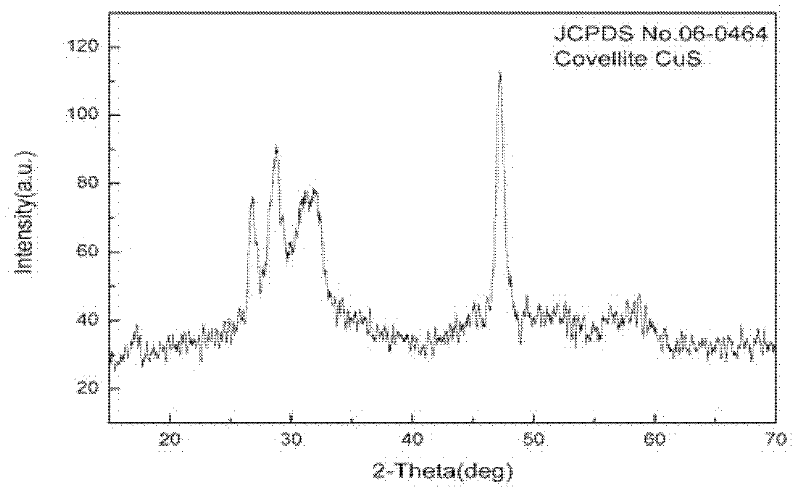
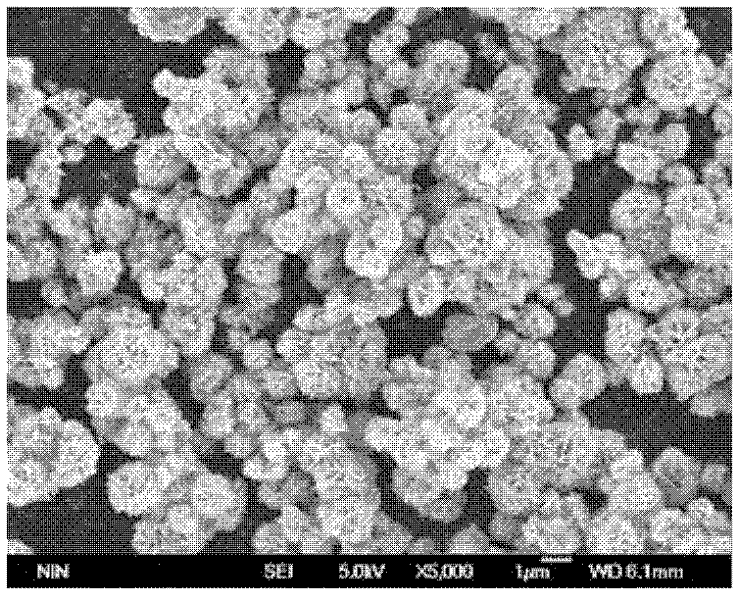
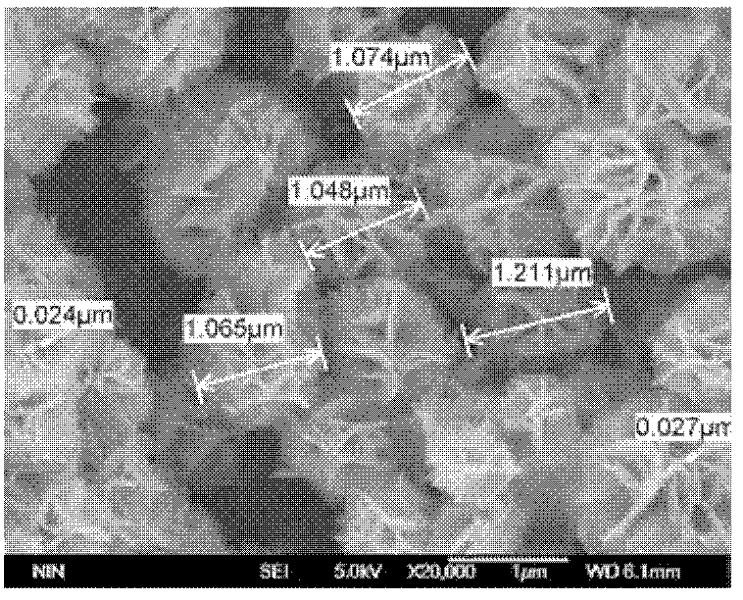
Method for preparing flower-shaped copper sulfide (CuS) nanocrystal
A copper sulfide and nanocrystal technology, applied in the field of semiconductor nanocrystals, can solve the problems of high energy consumption, long reaction time, environmental pollution, etc., and achieve the effects of low energy consumption, short reaction period and large surface area
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] 1) Analytical pure copper nitrate trihydrate Cu(NO 3 ) 2 ·3H 2 O dissolved in deionized water to make Cu 2+ A transparent solution A with a concentration of 0.04mol / L;
[0020] 2) Add analytically pure thiourea (SC(NH 2 ) 2 ), so that Cu in the solution 2+ : SC(NH 2 ) 2 The molar ratio of is 1: 1.5, obtains solution B;
[0021] 3) Add analytically pure cetyltrimethylammonium bromide (CTAB) to solution B, so that the concentration of CTAB in the solution is 0.005mol / L to form precursor solution C;
[0022] 4) Pour the precursor solution C into a microwave hydrothermal reactor with a filling degree of 50%, then seal the reactor, put it into a microwave hydrothermal reactor with temperature and pressure dual control, and select the temperature control mode to react at 140°C 5min, naturally cool to room temperature after the reaction finishes;
[0023] 5) Turn on the hydrothermal reaction kettle, collect the product by centrifugation, wash it with deionized water ...
Embodiment 2
[0026] 1) Analytical pure copper sulfate pentahydrate (CuSO 4 ·5H 2 O) dissolved in deionized water to make Cu 2+ A transparent solution A with a concentration of 0.06mol / L;
[0027] 2) Add analytically pure thiourea (SC(NH 2 ) 2 ), so that Cu in the solution 2+ : SC(NH 2 ) 2 The molar ratio of is 1:2, obtains solution B;
[0028] 3) Add analytically pure cetyltrimethylammonium bromide (CTAB) to solution B, so that the concentration of CTAB in the solution is 0.002mol / L, forming precursor solution C;
[0029] 4) Pour the precursor solution C into a microwave hydrothermal reactor with a filling degree of 60%, then seal the reactor, put it into a microwave hydrothermal reactor with temperature and pressure dual control, and select the temperature control mode to react at 130°C 20min, naturally cool to room temperature after the reaction finishes;
[0030] 5) Turn on the hydrothermal reaction kettle, collect the product by centrifugation, wash it with deionized water and...
Embodiment 3
[0033] 1) Analytical pure copper nitrate trihydrate Cu(NO 3 ) 2 ·3H 2 O dissolved in deionized water to make Cu 2+ A transparent solution A with a concentration of 0.08mol / L;
[0034] 2) Add analytically pure thiourea (SC(NH 2 ) 2 ), so that Cu in the solution 2+ : SC(NH 2 ) 2 The molar ratio of is 1: 2.5, obtains solution B;
[0035]3) Add analytically pure cetyltrimethylammonium bromide (CTAB) to solution B, so that the concentration of CTAB in the solution is 0.008mol / L to form precursor solution C;
[0036] 4) Pour the precursor solution C into the microwave hydrothermal reaction kettle, the filling degree is 50%, then seal the reaction kettle, put it into the microwave hydrothermal reaction instrument with dual temperature and pressure control, select the pressure control mode to react at 0.5Mpa 30min, naturally cool to room temperature after the reaction finishes;
[0037] 5) Turn on the hydrothermal reaction kettle, collect the product by centrifugation, wash ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com