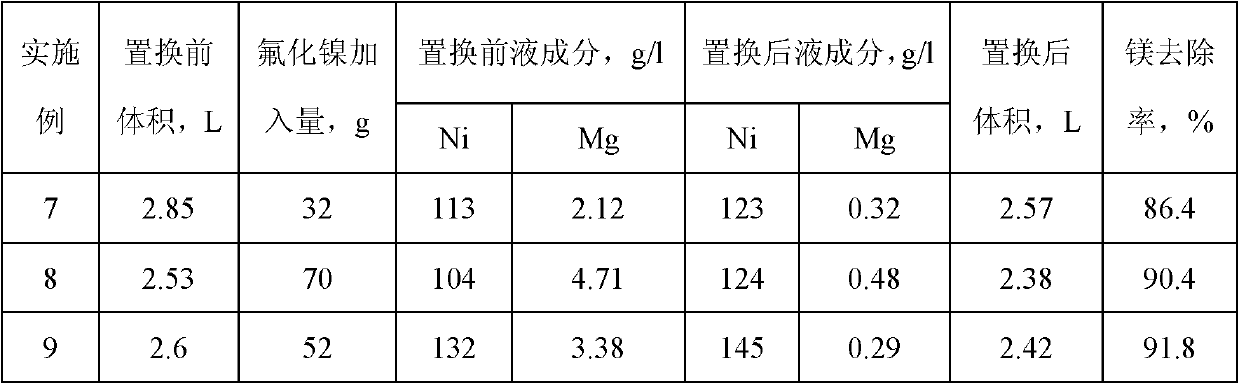
Method for removing calcium-magnesium impurities from nickel sulfate solution
A nickel sulfate and solution technology, applied in nickel sulfate and other directions, can solve the problems of long time, large consumption of fluoride salt, low solubility of calcium sulfate, etc., and achieve the effect of reducing dosage, high nickel yield and good impurity removal effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0038] The method of the present invention will be further explained and illustrated by the following examples, but it should be noted that these examples are only illustrative and cannot be used to limit the protection scope of the present invention.
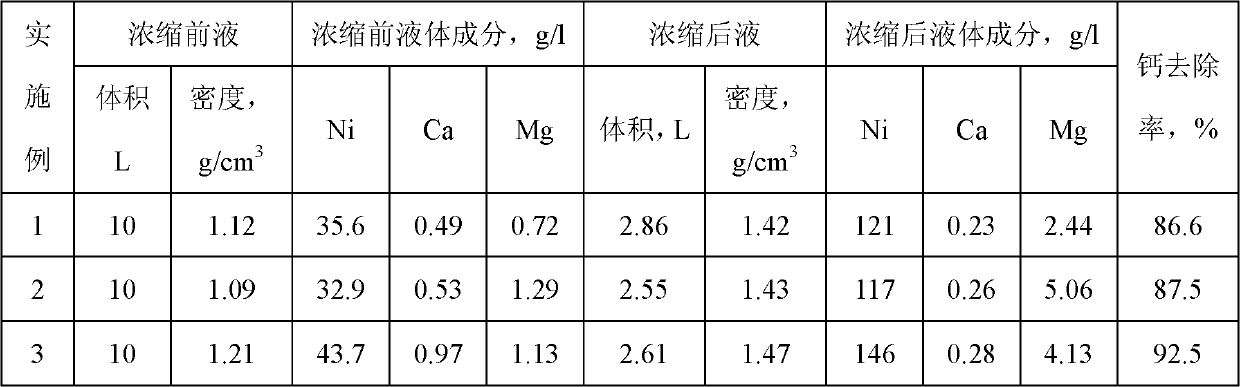
[0039] Embodiment 1 concentrates decalcification
[0040] The density of 10L is 1.12g / cm 3 The nickel sulfate solution after iron removal is heated and concentrated to a density of 1.42g / cm 3 , Calcium sulfate solid precipitated out after cooling. Filter to obtain 2.86L of nickel sulfate filtrate, analyze the change of impurities in the solution, and find that the removal rate of calcium reaches 86.6%.
[0041] Example 2-3 Concentrated Calcium Removal
[0042] Above embodiment is carried out by the step and method of embodiment 1, difference is the density of solution and the removal rate of calcium, and detailed result is listed in table 1.
[0043] Table 1 Summary of effects of concentrated decalcification
[0044]
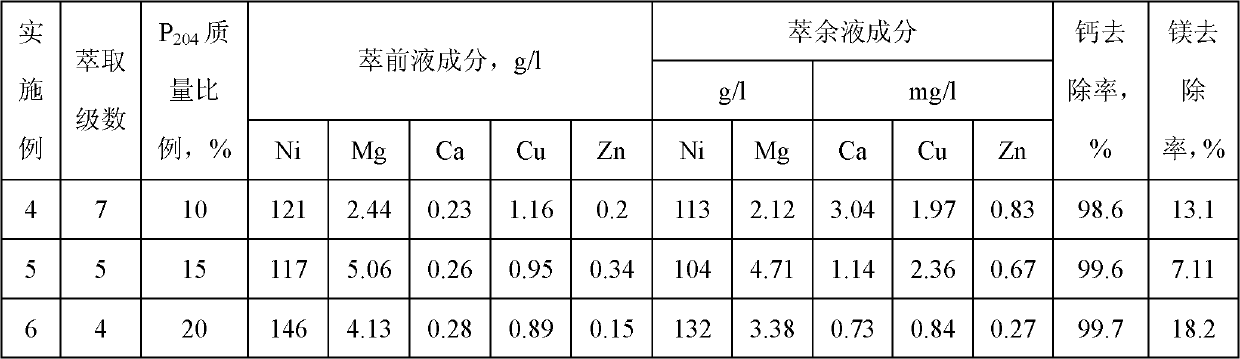
Embodiment 1
[0046] The 2.86L nickel sulfate filtrate pH value that embodiment 1 obtains is controlled at 4.1, and organic phase is by the P as extraction agent 204 and kerosene as diluent, where P 204 The mass ratio in the organic phase is 10%, the saponification rate is 50%, the ratio of the organic phase to the water phase is 1:1, after 7 stages of countercurrent contact extraction, 2.85L qualified raffinate is obtained, and the organic phase is loaded with 0.2N Sulfuric acid is used for 5-stage nickel washing to make nickel enter the water phase; 2N hydrochloric acid is used for 3-stage anti-copper zinc to make copper, zinc, calcium and magnesium enter the water phase; 6N hydrochloric acid is used for 2-stage anti-iron to extract iron from the organic phase. The results showed that the magnesium content in the raffinate decreased to 2.12g / l, the removal rate was 13.1%, and the calcium content decreased to 3.04mg / l, the removal rate was 98.6%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com