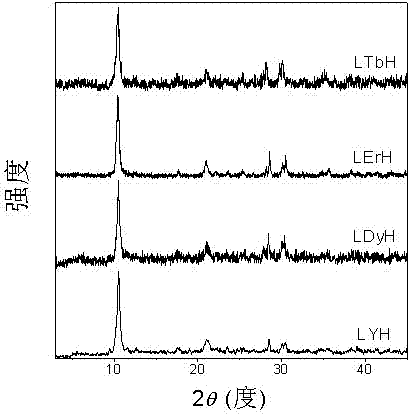
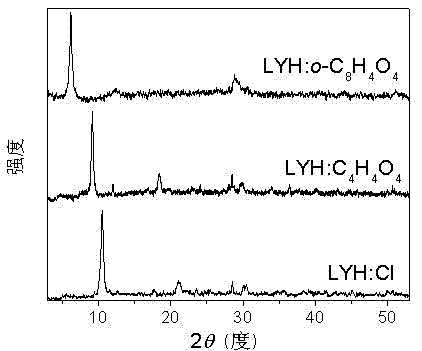
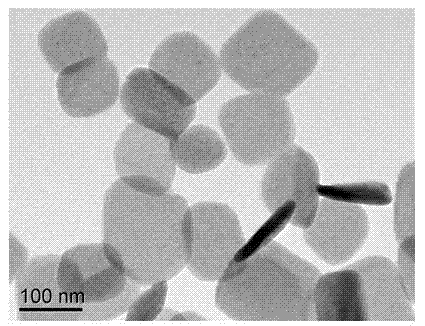
Nano lamellar compound rare-earth hydroxide and preparation method thereof
A rare earth hydroxide, layered technology, applied in chemical instruments and methods, luminescent materials, etc., to achieve the effects of good crystal shape, good dispersion and high particle purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] (1) Dissolve yttrium chloride and surfactant polyethyleneimine in deionized water respectively, the concentration of yttrium chloride is 0.1 mol / L, and the volume ratio of polyethyleneimine mass to solvent is 1 g:50 mL ;
[0042] (2) Add 1 mL of yttrium chloride solution, 15 mL of ethanol, and 1 mL of polyethyleneimine solution into the reaction kettle in sequence, and vigorously stir for 5 to 10 minutes at room temperature under the protection of a nitrogen atmosphere to make the solutions evenly mixed.
[0043] (3) Move the reactor into a preheated furnace for crystallization at 80 oC for 2.5 h.
[0044] (4) Allow the reactor to cool down at room temperature, centrifuge the resulting solution, wash with deionized water and absolute ethanol three times, and then dissolve in 5 mL of deionized water to obtain an aqueous solution of yttrium nanolayered hydroxide.
Embodiment 2
[0046] (1) Dissolve yttrium chloride and surfactant polyethyleneimine in deionized water respectively, the concentration of yttrium chloride is 0.5 mol / L, and the volume ratio of polyethyleneimine mass to solvent is 1 g:50 mL ;
[0047] (2) Add 1 mL of yttrium chloride solution, 15 mL of ethanol, and 5 mL of polyethyleneimine solution into the reaction kettle in sequence, and vigorously stir for 5 to 10 minutes at room temperature under the protection of a nitrogen atmosphere to make the solutions evenly mixed.
[0048] (3) Move the reactor into a preheated furnace for crystallization at 100 oC for 2.5 h.
[0049] (4) Allow the reactor to cool down at room temperature, centrifuge the resulting solution, wash with deionized water and absolute ethanol three times, and then dissolve in 5 mL of deionized water to obtain an aqueous solution of yttrium nanolayered hydroxide.
Embodiment 3
[0051] (1) Dissolve yttrium chloride and surfactant polyethyleneimine in deionized water respectively, the concentration of yttrium chloride is 0.5 mol / L, and the volume ratio of polyethyleneimine mass to solvent is 1 g:50 mL ;
[0052] (2) Add 1 mL of yttrium chloride solution, 15 mL of ethanol, and 5 mL of polyethyleneimine solution into the reaction kettle in sequence, and vigorously stir for 5 to 10 minutes at room temperature under the protection of a nitrogen atmosphere to make the solutions evenly mixed.
[0053] (3) Move the reactor into a preheated furnace for crystallization at 160 oC for 2.5 h.
[0054] (4) Allow the reactor to cool down at room temperature, centrifuge the resulting solution, wash with deionized water and absolute ethanol three times, and then dissolve in 5 mL of deionized water to obtain an aqueous solution of yttrium nanolayered hydroxide.
[0055] Ion exchange of yttrium nanolayered hydroxides: Typically, one part of the prepared LYH is dissolve...
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com