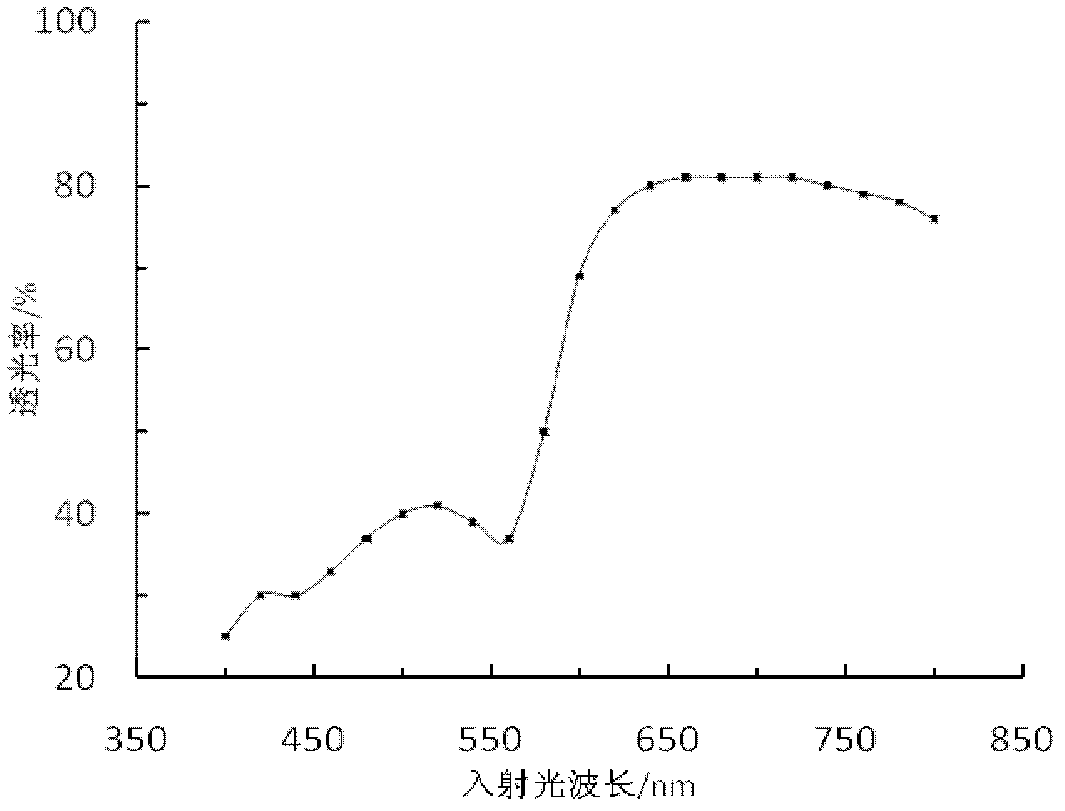
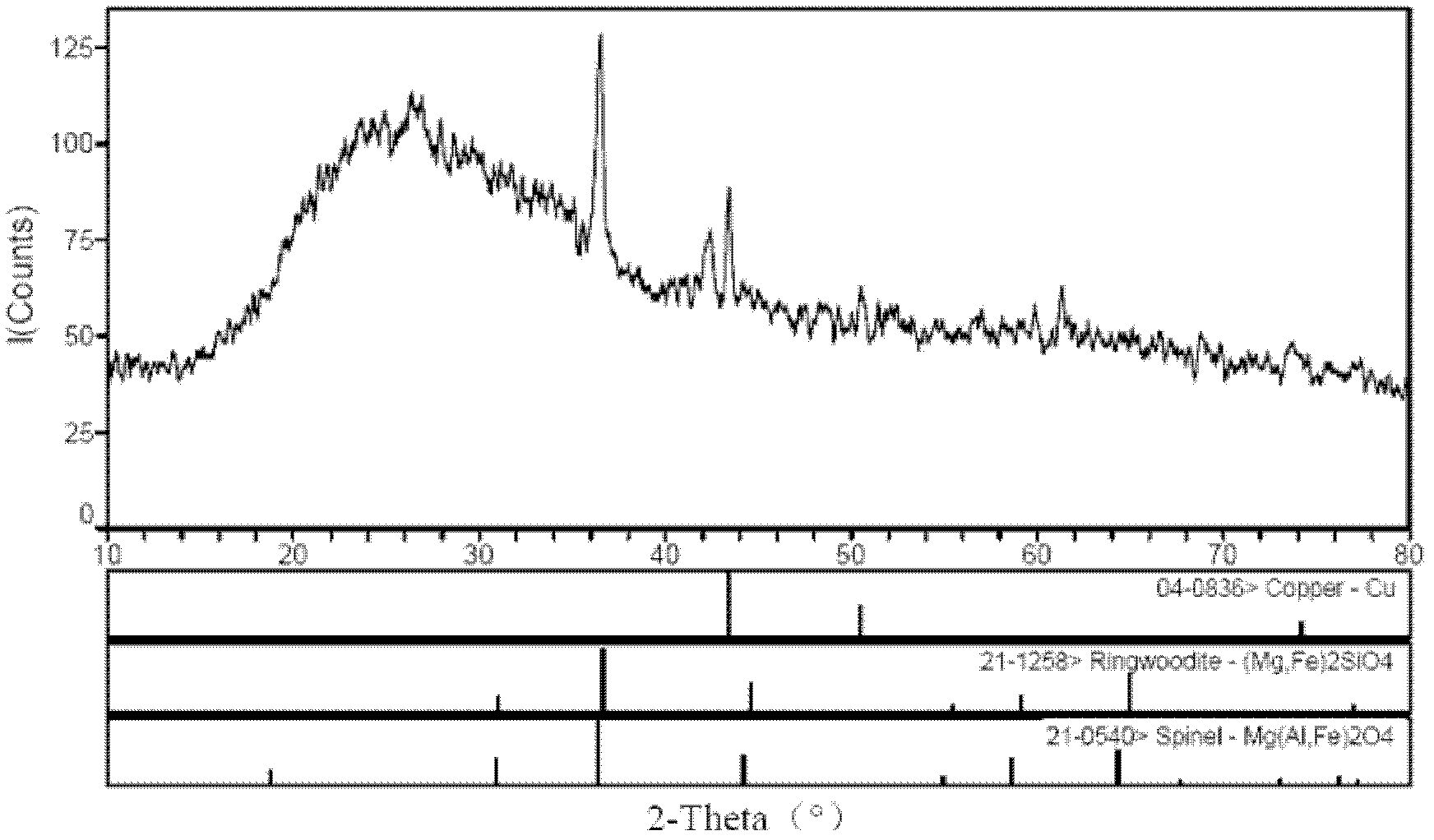
Preparation method of red hard glass
A hard glass, red technology, applied in the field of preparation of red hard glass, can solve the problems such as the inability to obtain uniform colored red glass, the lack of formulation and process system, the long holding time, etc., and the short firing time is achieved. , The process is easy to control and the quality is stable.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Embodiment 1: the first step, preparation of colored slurry:
[0019] First, press KNO 3 : K 2 SO 4 :KOH:Cu 2 O: SnO: FeO: CoO: FeSO 4 ·7H 2 O: water = 6: 3: 1: 8: 6: 3: 1: 8: 10 mass ratio to take KNO 3 、K 2 SO 4 , KOH, Cu 2 O, SnO, FeO, CoO, FeSO 4 ·7H 2 O and water;
[0020] Then, put the raw materials except water into an agate mortar and mix them evenly, then add them into a ball mill jar, then add water for ball milling, and pass through a 300-mesh standard sieve after the ball milling to get the colored slurry;
[0021] The second step, coating and firing:
[0022] First, clean the glass surface of the hard instrument and dry it for later use;
[0023] Then, evenly coat the coloring slurry on the surface of the hard glass with a coating thickness of 0.5mm, then put it in an oven at 110°C for 30 minutes, take it out and put it in a muffle furnace, follow the following process System firing: raise the temperature from room temperature to 480°C at a he...
Embodiment 2
[0025] Embodiment 2: the first step, preparation of colored slurry:
[0026] First, press KNO 3 : K 2 SO 4 :KOH:Cu 2 O: SnO: FeO: CoO: FeSO 4 ·7H 2 O: water = 8: 3: 2: 12: 7: 4: 1: 7: 11 mass ratio to take KNO 3 、K 2 SO 4 , KOH, Cu 2 O, SnO, FeO, CoO, FeSO 4 ·7H 2 O and water;
[0027] Then, put the raw materials except water into an agate mortar and mix them evenly, then add them into a ball mill jar, then add water for ball milling, and pass through a 300-mesh standard sieve after the ball milling to get the colored slurry;
[0028] The second step, coating and firing:
[0029] First, clean the glass surface of the hard instrument and dry it for later use;
[0030] Then, evenly coat the colored slurry on the surface of the hard glass with a coating thickness of 0.6mm, then put it in an oven at 110°C for 30 minutes, take it out and put it in a muffle furnace, follow the following process System firing: heat up from room temperature to 480°C at a heating rate of ...
Embodiment 3
[0031] Embodiment 3: the first step, preparation of colored slurry:
[0032] First, press KNO 3 : K 2 SO 4 :KOH:Cu 2 O: SnO: FeO: CoO: FeSO 4 ·7H 2 O: water = 5: 4: 1: 10: 8: 3: 1: 6: 9 mass ratio to take KNO 3 、K 2 SO 4 , KOH, Cu 2 O, SnO, FeO, CoO, FeSO 4 ·7H 2 O and water;
[0033] Then, put the raw materials except water into an agate mortar and mix them evenly, then add them into a ball mill jar, then add water for ball milling, and pass through a 300-mesh standard sieve after the ball milling to get the colored slurry;
[0034] The second step, coating and firing:
[0035] First, clean the glass surface of the hard instrument and dry it for later use;
[0036] Then, evenly coat the coloring slurry on the surface of the hard glass with a coating thickness of 0.8mm, then put it in an oven at 110°C for 30 minutes, take it out and put it in a muffle furnace, follow the following process System firing: raise the temperature from room temperature to 480°C at a he...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com