Synthetic method of cyhalofop-butyl active compound
A technology of cyhalofop-butyl and a synthesis method is applied in the field of synthesis of cyhalofop-butyl, which can solve the problems of difficult treatment, high cost of raw materials, and many three wastes, and achieves difficult racemization reaction, lower reaction temperature, and reaction selection. high sex effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
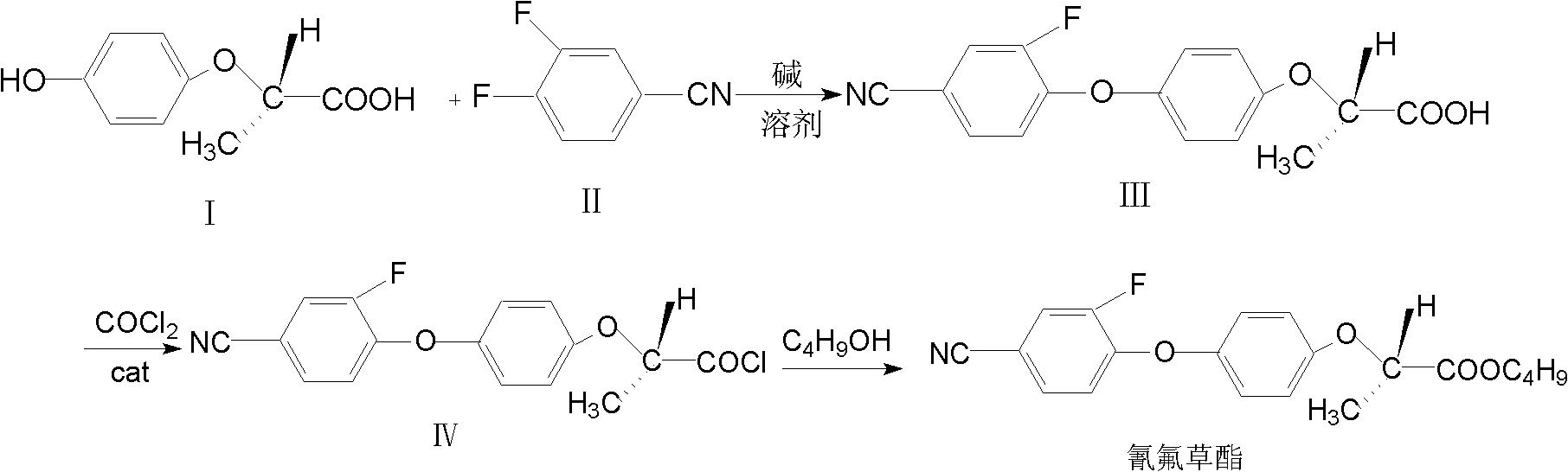
[0024] (1) Drop 500ml of ethylene glycol dimethyl ether into a 1000ml four-necked flask, (R)-4-hydroxyphenoxypropionic acid (I) 91g (0.5mol) [R / S=99 / 1], anhydrous Potassium carbonate 173.5g (2.5mol), polyethylene glycol-4002g, 3,4-difluorobenzonitrile (II) 69.5g (0.5mol). The temperature was raised to 85-90° C., and the reaction was kept for 6 hours, and the reaction was completed. Cool down to room temperature, filter, wash the filter cake with 100ml of ethylene glycol dimethyl ether, combine the filtrate and washing liquid, remove the solvent of ethylene glycol dimethyl ether under reduced pressure, add 300ml of water, and adjust the pH value to 1~2 with 10% hydrochloric acid 2. Add 500ml of toluene for extraction and separation, and dehydrate under reflux to obtain intermediate (R)-2-[4-(2-fluoro-4-nitrile)-phenoxy]-propionic acid (III) in toluene.
[0025] (2) Cool the (R)-2-[4-(2-fluoro-4-nitrile)-phenoxy]-propionic acid (III) toluene solution prepared above to 50°C, and...
Embodiment 2
[0028] (1) drop 500ml of dimethyl sulfoxide into a 1000ml four-necked flask, (R)-4-hydroxyphenoxypropionic acid (I) 91g (0.5mol) [R / S=99 / 1], anhydrous potassium carbonate 173.5g (2.5mol), 2g of tetrabutylammonium bromide, 69.5g (0.5mol) of 3,4-difluorobenzonitrile (II), heated up to 85°C-90°C, kept for 10 hours, and the reaction was completed. Cool down to room temperature, filter, wash the filter cake with 100ml dimethyl sulfoxide, combine the filtrate and washing liquid, remove the solvent dimethyl sulfoxide under reduced pressure, add 300ml of water, adjust the pH value to 1-2 with 10% hydrochloric acid, add 500ml Extract and separate with toluene, and dehydrate under reflux to obtain intermediate (R)-2-[4-(2-fluoro-4-nitrile)-phenoxy]-propionic acid (III) in toluene.
[0029] Photochemical and esterification reactions are the same as steps (2) and (3) in Example 1. The obtained cyhalofop-ethyl technical product was 150 g, the chemical content was 94.8% [R / S=97 / 3], and the...
Embodiment 3
[0031] (1) The synthesis of intermediate (R)-2-[4-(2-fluoro-4-nitrile)-phenoxy]-propionic acid (III) is the same as step (1) in Example 1.
[0032] (2) Add 1 g of catalyst DMF to the toluene solution of (R)-2-[4-(2-fluoro-4-nitrile)-phenoxy]-propionic acid (III) prepared above. Raise the temperature to 80°C-90°C, and pass through phosgene at a speed of 1200ml / min-1400ml / min. After passing through the light for 2 hours, pass through nitrogen to drive away the residual phosgene and hydrogen chloride gas to obtain the intermediate (R)- 2-[4-(2-Fluoro-4-cyano)-phenoxy]-propionyl chloride (IV) in toluene.
[0033] (3) Esterification is the same as step (3) in Example 1. The obtained cyhalofop-ethyl technical product was 151g, the chemical content was 96.8% [R / S=75 / 25], and the yield was 84.6% (calculated as 3,4-difluorobenzonitrile, the final percent). Appearance: white solid.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com