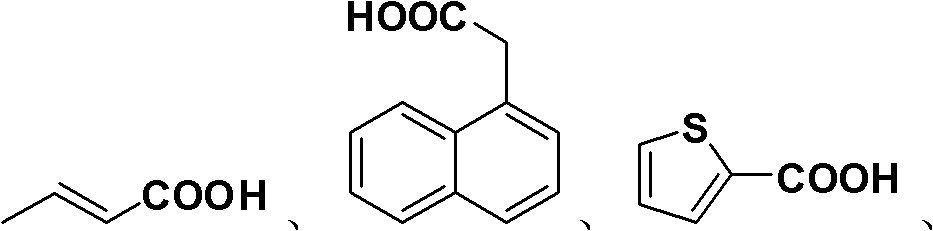
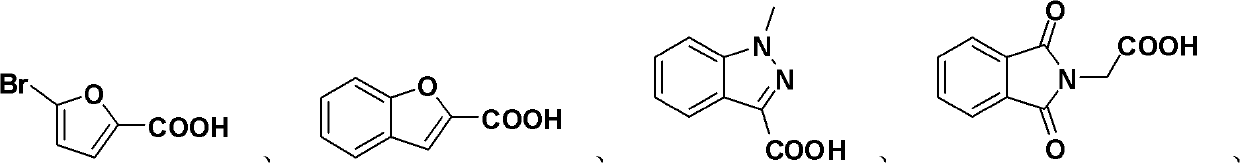
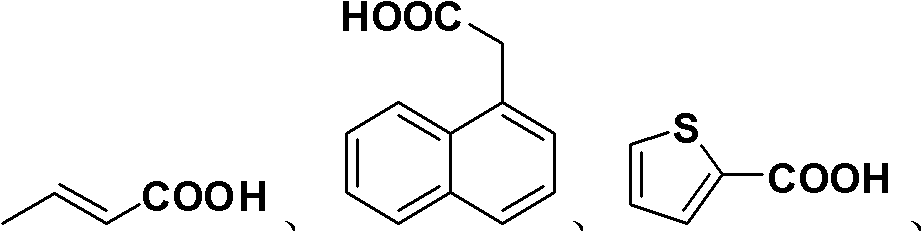
Preparation method of allyl esters
A technology of allyl esters and olefins, applied in the field of organic synthesis, can solve the problems of limited large-scale application, troublesome preparation of reactants, narrow use range of substrates, etc., to achieve improved utilization efficiency, wide range of use, and excellent yield Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024]
[0025] Fill the reaction bottle with Bu 4 NI (20mol%), compound 1a (2mmol, 313.2mg), tert-butyl hydroperoxide (TBHP) (0.4mL), cyclohexene (0.8mL), benzene 8mL; After heating for about 8 hours, it was quenched with saturated sodium sulfite, extracted with ethyl acetate (40 mL×3), dried over anhydrous sodium sulfate, and the oxidation product 3a was obtained by simple column chromatography with a yield of 88%. 1 H NMR (400MHz, CDCl 3 )δ7.98(d, J=8.5Hz, 2H), 7.40(d, J=8.5Hz, 2H), 6.03-5.99(m, 1H), 5.84-5.80(m, 1H), 5.50-5.49(m , 1H), 2.17-1.93(m, 3H), 1.89-1.78(m, 2H), 1.75-1.67(m, 1H); 13 C NMR (100MHz, CDCl 3 )δ165.2, 139.0, 132.9, 130.9, 129.1, 128.5, 125.4, 68.8, 28.3, 24.8, 18.8.MS: Anal.Calcd.For C 13 h 13 35 ClO 2 :236,C 13 h 13 37 ClO 2 : 238, Found: 236 ( 35 Cl), 238( 37 Cl).IR(KBr, cm -1 ): v 1716.
Embodiment 2
[0027]
[0028] Fill the reaction bottle with Bu 4 NI (20 mol%), compound 1a (2 mmol, 313.2 mg), TBHP (0.4 mL), cyclohexene (0.8 mL), cyclohexane 8 mL. Then the system was heated in the air at 80°C for about 8 hours, quenched with saturated sodium sulfite, extracted with ethyl acetate (40mL×3), dried over anhydrous sodium sulfate, and the oxidation product 3a was obtained by simple column chromatography , the yield was 68%. 1 H NMR (400MHz, CDCl 3 )δ7.98(d, J=8.5Hz, 2H), 7.40(d, J=8.5Hz, 2H), 6.03-5.99(m, 1H), 5.84-5.80(m, 1H), 5.50-5.49(m , 1H), 2.17-1.93(m, 3H), 1.89-1.78(m, 2H), 1.75-1.67(m, 1H); 13 C NMR (100MHz, CDCl 3 )δ165.2, 139.0, 132.9, 130.9, 129.1, 128.5, 125.4, 68.8, 28.3, 24.8, 18.8.MS: Anal.Calcd.For C 13 h 13 35 ClO 2 :236,C 13 h 13 37ClO 2 : 238, Found: 236 ( 35 Cl), 238( 37 Cl).IR(KBr, cm -1 ): v 1716.
Embodiment 3
[0030]
[0031] Fill the reaction bottle with Bu 4 NI (20mol%), compound 1a (2mmol, 313.2mg), TBHP (0.4mL), cyclohexene (0.8mL), acetocyanide 8mL. Then the system was heated in the air at 80°C for about 8 hours, quenched with saturated sodium sulfite, extracted with ethyl acetate (40mL×3), dried over anhydrous sodium sulfate, and the oxidation product 3a was obtained by simple column chromatography , the yield was 50%. 1 HNMR (400MHz, CDCl 3 )δ7.98(d, J=8.5Hz, 2H), 7.40(d, J=8.5Hz, 2H), 6.03-5.99(m, 1H), 5.84-5.80(m, 1H), 5.50-5.49(m , 1H), 2.17-1.93(m, 3H), 1.89-1.78(m, 2H), 1.75-1.67(m, 1H); 13 C NMR (100MHz, CDCl 3 )δ165.2, 139.0, 132.9, 130.9, 129.1, 128.5, 125.4, 68.8, 28.3, 24.8, 18.8.MS: Anal.Calcd.For C 13 h 13 35 ClO 2 :236,C 13 h 13 37 ClO 2 : 238, Found: 236 ( 35 Cl), 238( 37 Cl).IR(KBr, cm -1 ): v 1716.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com