Method for purifying and refining dimethoxy taxane compound
A dimethoxytaxane, purification and refining technology, applied in the direction of organic chemistry, can solve the problems of difficult separation of reaction by-products and compounds, and inability to obtain compounds, so as to improve reaction yield and product purity, and facilitate industrialization The effect of simple production and operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] 30.0 grams of the crude product (purity is 73.8%) obtained according to the preparation method of the compound (1) proposed in the patent CN 1179776A was placed on a chromatographic column (20 cm in inner diameter, 80 cm in height) equipped with 1.2 kg of 400-800 mesh silica gel. centimeter), and use a mixed solvent of petroleum ether / ethyl acetate to elute, collect the eluate containing compound (1), and concentrate to dryness at 40°C under reduced pressure (2.7KPa) to obtain 12.0 grams of crude product. The purity was 89.4%.
[0019] Put the above crude product into tetrahydrofuran (120mL), add acetic anhydride (1.2mL), cerium trichloride heptahydrate (0.94g), react at room temperature for 24 hours, pour into ice water (500mL), add ethyl acetate ( 200mL), stirred thoroughly for 30 minutes. The organic phase was separated, washed with saturated brine (1000 mL×3), dried over anhydrous sodium sulfate, and concentrated to dryness at 40° C. under reduced pressure (2.7 KPa...
Embodiment 2
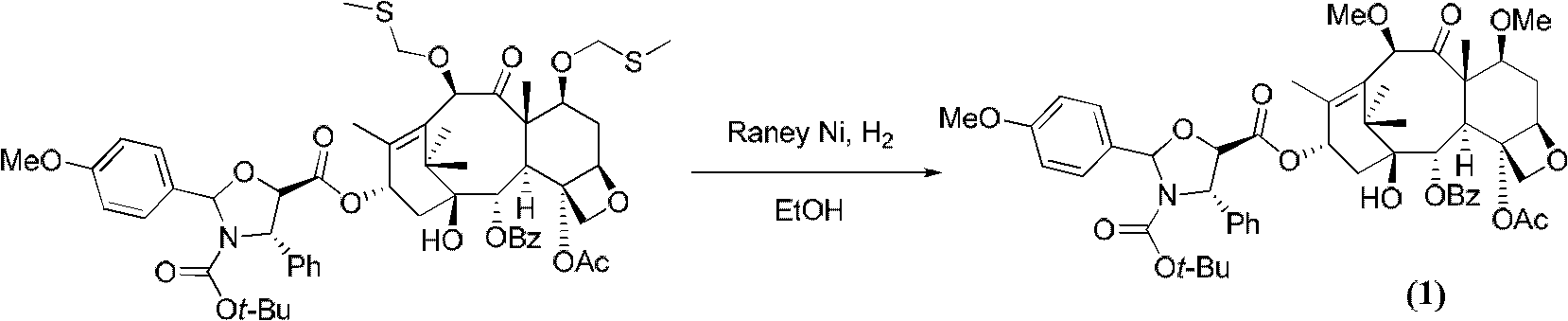
[0022] 30.0 grams of the crude product (purity is 73.8%) obtained according to the preparation method of the compound (1) proposed in the patent CN 1179776A was placed on a chromatographic column (20 cm in inner diameter, 80 cm in height) equipped with 1.2 kg of 400-800 mesh silica gel. centimeter), and use a mixed solvent of petroleum ether / ethyl acetate to elute, collect the eluate containing compound (1), and concentrate to dryness at 40°C under reduced pressure (2.7KPa) to obtain 12.0 grams of crude product. The purity was 89.4%.
[0023] Put the above crude product into dichloromethane (60mL), add pyridine (1.0mL), add 2,2,2-trichloroethoxycarbonyl chloride (0.87mL) dropwise at 0°C, after dropping, react at room temperature for 24 hours , poured into ice water (500 mL), added dichloromethane (400 mL), and stirred thoroughly for 30 minutes. The organic phase was separated, washed with saturated brine (1000 mL×3), dried over anhydrous sodium sulfate, and concentrated to dr...
Embodiment 3
[0026] 30.0 grams of the crude product (purity is 73.8%) obtained according to the preparation method of the compound (1) proposed in the patent CN 1179776A was placed on a chromatographic column (20 cm in inner diameter, 80 cm in height) equipped with 1.2 kg of 400-800 mesh silica gel. centimeter), and use a mixed solvent of petroleum ether / ethyl acetate to elute, collect the eluate containing compound (1), and concentrate to dryness at 40°C under reduced pressure (2.7KPa) to obtain 12.0 grams of crude product. The purity was 89.4%.
[0027] Put the above crude product into dimethylsulfoxide (24.0mL), add acetic anhydride (16.0mL), acetic acid (1.0mL), react at room temperature for 96 hours, pour into ice water (1000mL), add ethyl acetate (800mL ), fully stirred for 30 minutes. The organic phase was separated, washed with saturated aqueous sodium bicarbonate (1000 mL×3), washed with saturated brine (1000 mL×3), dried over anhydrous sodium sulfate, and concentrated to dryness...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com