Fusion protein containing guide peptide and GnRH-PE39KDEL as well as nucleic acid and application thereof
A fusion protein and guide peptide technology, applied in the field of bioengineering, can solve the problems of weak target tissue-specific killing effect, poor infiltration of large solid tumors, serious immune response, etc., and achieves less stringent reaction conditions, high speed, and temperature adaptability. wide effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Example 1 Obtaining of GnRH-PTD-PE39KDEL fusion protein gene sequence
[0027] According to the known amino acid sequence of GnRH-PE39KDEL, the GnRH-PE39KDEL gene sequence (completed by Sangon Bioengineering (Shanghai) Co., Ltd.) was synthesized using the preferred codons of Escherichia coli. C-terminus plus a restriction enzyme site.
[0028] The PCR method was used to operate the gene sequence of the whole gene synthesis PE39KDEL, and three primers were designed:
[0029] MiddleCTDF1
[0030] 5'TACAACGCTGGTCGTCGTGCTCGTCGTCGTCGTCGTCGTCACTTCCCGGAAGGTGG
[0031] MiddleCTDF2
[0032] 5’ GGGATCCGTGGAAGGTCGCGAACACTGGTCTTACGGTCTGCGTCCGGGTTACAACGCTGGTCGTCG
[0033] GnRHR 5' GAATTCTCACAGTTCGTCTTTCGG
[0034] A PTD sequence was introduced at the C-terminal of GnRH of GnRH-PE39KDEL, and a Factor Xa protease cleavage site was introduced at the N-terminal of GnRH-PTD-PE39KDEL to obtain the nucleic acid sequence SEQ ID NO:1 encoding the fusion protein of SEQ ID NO:2.
Embodiment 2
[0036] 2.1 Construction of GnRH-PTD-PE39KDEL gene recombinant plasmid:
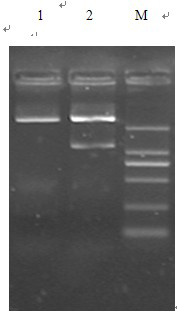
[0037] Extract the plasmid pET-His with Bam H I and Eco R I double digestion, recovery of 2.8 kb linear plasmid;
[0038] The GnRH-PTD-PE39KDEL gene fragment obtained in the step 1 was used Bam H I and Eco R I double enzyme digestion, add equal volume of phenol: chloroform: isoamyl alcohol (volume ratio 25:24:1) for extraction, 3 times volume of absolute ethanol precipitation to recover the digested fragment;
[0039] Ligate the fragments of the digested products recovered above with T4 DNA ligase, and the ligation reaction system is 25 μl:
[0040]
[0041] The ligation was performed overnight at 16°C, and the ligation product was the pET-His-GnRH-PTD-PE39KDEL recombinant plasmid.
[0042] 2.2 The recombinant plasmid pET-His-GnRH-PTD-PE39KDEL was transformed into Escherichia coli Top 10F to verify the recombination results:
[0043] Take 1 μl of pET-His-GnRH-PTD-PE39KDEL recombinant plasmid,...
Embodiment 3
[0044] Example 3 Construction and screening of engineering strains expressing GnRH-PTD-PE39KDEL
[0045] 3.1 Transformation of recombinant plasmid pET-His-GnRH-PTD-PE39KDEL into expression strain BL21(DE3)plysS
[0046] Take 1 μl of the pET-His-GnRH-PTD-PE39KDEL plasmid constructed in 2.1 above, dilute it 10 times and transform it directly E. coli The expression strain BL21(DE3)plysS competent cells were spread on LB+Amp plates and cultured upside down at 37°C overnight.
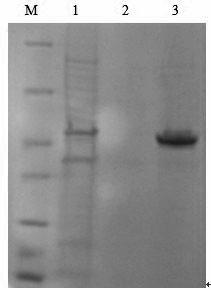
[0047] 3.2 Screen the engineering strain GnRH-PTD-PE39KDEL / pET-His expressing GnRH-PTD-PE39KDEL
[0048] Pick 4 monoclonal colonies from the above LB plate, shake overnight culture in 2ml LB+Amp liquid medium at 37°C, take 20 μl overnight culture medium and add it to 2 ml YTA+Amp liquid medium for transfer culture, 37 Cultivate with shaking at °C for 3 hours until the OD600 is between 0.5 and 0.7, then add IPTG to a final concentration of 0.3mM, and induce expression at 30°C for 3-8 hours with sha...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com