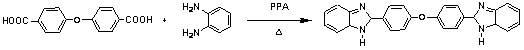N-substituted polybenzimidazole amide compound and preparation method thereof
A technology of benzimidazole and compound, which is applied in the field of N-substituted polybenzimidazole amide compound and its preparation, can solve the problems of affecting heat resistance level and lowering stability, and achieves the effect of low preparation cost and simple operation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Embodiment 1 The polycondensation synthesis of N-substituted polybenzimidazole amide compound
[0029] (1) In a 25mL three-necked flask, add 0.01mol terephthalic acid, 0.022mol o-phenylenediamine, 8mL polyphosphoric acid, 2 Under the protection of , the reaction was stirred at 200°C for 6h. After the solution is cooled, pour it into 100mL of cold distilled water, add dropwise 15% NaOH solution, neutralize to pH=8, and generate a large amount of precipitate. Suction filtration, washing with water, and drying gave solid crude product. The crude product was dissolved in absolute ethanol for recrystallization, activated carbon decolorization and vacuum drying at 60°C to obtain the bis(benzimidazole) intermediate- .
[0030]
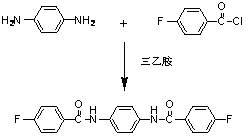
[0031] (2) In a 50mL three-necked flask, add 0.01mol p-phenylenediamine, 0.05mol 4-fluorobenzoyl chloride, 20mL triethylamine, in N 2 Under the protection of , the reaction was stirred overnight in an ice-water bath. Then the mixed solution wa...
Embodiment 2
[0037] Embodiment 2 The polycondensation synthesis of N-substituted polybenzimidazole amide compound
[0038] ⑴In a 25mL three-necked flask, add 0.01mol 4'4dicarboxylic acid diphenyl ether, 0.022mol o-phenylenediamine, 8mL polyphosphoric acid, in N 2 Under the protection of , the reaction was stirred at 200°C for 6h. After the solution is cooled, pour it into 100mL of cold distilled water, add dropwise 15% NaOH solution, neutralize to pH=8, and generate a large amount of green precipitate. Suction filtration, washing with water, and drying gave solid crude product. The crude product was dissolved in absolute ethanol for recrystallization, decolorized by activated carbon and vacuum dried at 60°C to obtain bis(benzimidazole) compound- .
[0039]
[0040] (2) In a 50mL three-necked flask, add 0.01mol 4'4-diaminodiphenyl ether, 0.05mol 4-fluorobenzoyl chloride, 20mL triethylamine, 2 Under the protection of , the reaction was stirred overnight in an ice-water bath. Then t...
Embodiment 3
[0043] Embodiment 3 The polycondensation synthesis of N-substituted polybenzimidazole amide compound
[0044] (1) In a 25mL three-necked flask, add 0.01mol 4'4 diphenyl sulfone dicarboxylate, 0.022mol o-phenylenediamine, 8mL polyphosphoric acid, in N 2 Under the protection of , the reaction was stirred at 200°C for 6h. After the solution is cooled, pour it into 100mL of cold distilled water, add dropwise 15% NaOH solution, neutralize to pH=8, and generate a large amount of green precipitate. Suction filtration, washing with water, and drying gave solid crude product. The crude product was dissolved in absolute ethanol for recrystallization, decolorized by activated carbon and vacuum dried at 60°C to obtain bis(benzimidazole) compound- .
[0045]
[0046] (2) In a 50mL three-necked flask, add 0.01mol 1,4-bis(4-aminophenoxy)benzene, 0.05mol 4-fluorobenzoyl chloride, 20mL triethylamine, in N 2 Under the protection of , the reaction was stirred overnight in an ice-water b...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Density | aaaaa | aaaaa |
| Glass transition temperature | aaaaa | aaaaa |
| Density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com