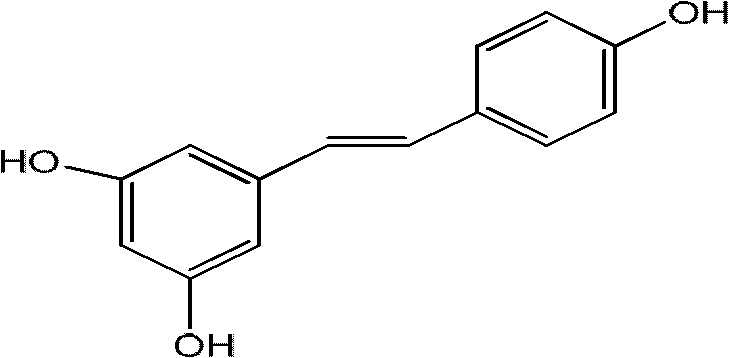
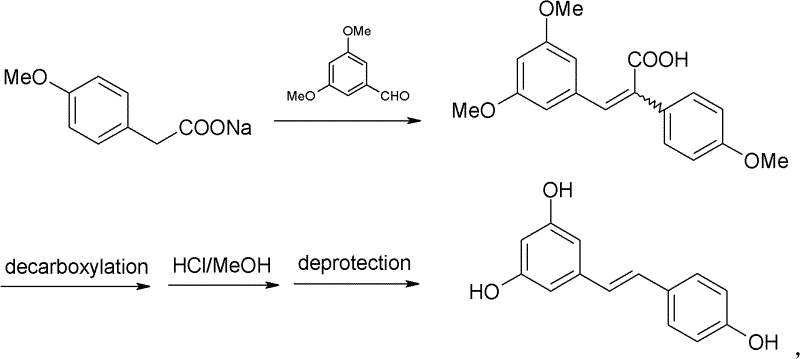
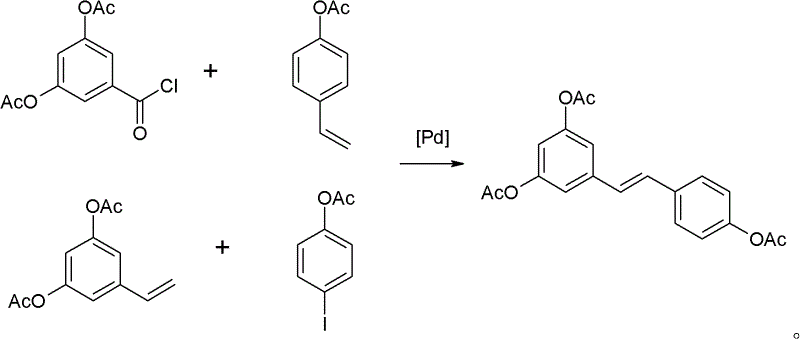
Process for synthesizing trimethoxyphenyl stilbene
A technology of trimethoxystilbene and dimethoxybenzyl alcohol, applied in the field of environment-friendly synthesis, can solve the problems of low yield and the like, and achieve the effects of low unit consumption, less pollution of three wastes, and easy availability of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] Add 84 grams of 3,5-dimethoxybenzyl alcohol (0.5mol), 150 milliliters of toluene and 73 grams of DMF (1mol) in a 500 milliliter three-necked flask, and cool to 10°C; 59.4 grams (0.2mol) of triphosgene are dissolved in Add 150 ml of toluene dropwise. During the dropwise addition, control the temperature below 15°C. After the dropwise addition, remove the ice-water bath and continue the reaction for 1 hour; let stand to separate the darker oil phase in the lower layer, and use a small amount of ice water in the upper toluene phase. and saturated sodium bicarbonate (30 ml each) were washed successively, and then concentrated to recover toluene to obtain 92.5 g of crude product.
[0053] The crude product is compared with the 3,5-dimethoxybenzyl chloride reference substance, and the HPLC peak position and the TLC spot position are consistent, indicating that the crude product is a crude product of 3,5-dimethoxybenzyl chloride.
[0054] TLC conditions: petroleum mystery: chl...
Embodiment 2
[0064] Add 92.5 grams of the crude product in Example 1 into 93 grams (0.75mol) of trimethyl phosphite and reflux for 10 hours until the oil bath temperature is 160°C ± 5°C and the internal temperature is 150°C. TLC detects that the reaction is complete and the product a is obtained .
[0065] The product a is compared with 3,5-dimethoxybenzyl phosphonic acid dimethyl ester, and the TLC spot position is consistent, indicating that the product a is 3,5-dimethoxybenzyl phosphonic acid dimethyl ester.
[0066] TLC conditions: Petroleum: chloroform: ethyl acetate = 1:1:0.1, volume ratio.
Embodiment 3
[0068] At room temperature, the product a in Example 2 was added to a methanol solution of 144.64 grams of sodium methoxide (wherein, the mass percent concentration of sodium methoxide was 28%, which was 0.75mol), stirred for 2 hours, and 68 grams of anisaldehyde ( 0.5mol), stirred at room temperature for 8 hours, heated to 80°C to recover most of methanol, and then distilled off residual methanol under reduced pressure. Be cooled to below 15 ℃, add dropwise 150 milliliters of volume percent concentration be the sulfuric acid aqueous solution of 25%, separate out product 128 grams, use 125 milliliters of volume percent concentration as the ethanol aqueous solution recrystallization of 95% to obtain 116.7 grams of white powder, yield 91 % (calculated as 3,5-dimethoxybenzyl alcohol), the purity is 99.2%.
[0069] The characterization data of the white powder are as follows: m.p.54°C-56°C, IR (cm -1 ): 3060, 1595, 1512, 1458, 1252, 1153, 961; m / z: 270, 255, 239, 147, 135, 90;
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com