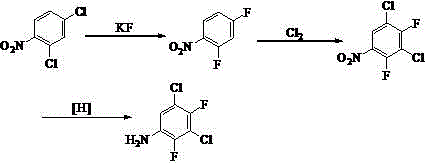
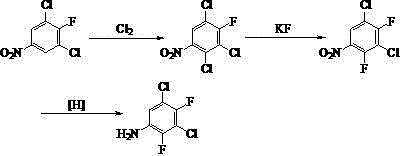
Synthetic method of 3, 5-dichloro-2, 4-difluoroaniline
A technology of difluoroaniline and synthesis method, which is applied in chemical instruments and methods, preparation of amino compounds, preparation of organic compounds, etc., can solve the problems of high cost, three wastes, and difficult availability of raw materials, and achieve low cost and avoid isomers , The effect of reducing the pollution of three wastes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Take 43.0 g of the distillation residue of the production process of 2,4-dichloro-3-fluoronitrobenzene (from an enterprise in Kaihua, Zhejiang Province to prepare 2,4-dichloro-3-fluoronitrobenzene by nitration of 2,6-dichlorofluorobenzene Distillation residue with batch number 20110912 in 2011 production process of benzene, in which 3,5-dichloro-4-fluoronitrobenzene content is 83.7%, 2,4-dichloro-3-fluoronitrobenzene content is 14.6%) and Dissolve 2.0 g of iodine in 80 mL of DMSO, raise the temperature to 140 °C and start flowing chlorine gas, react for 12 h, stop the chlorine flow, pour the reaction solution into ice water, wash the organic phase with NaOH aqueous solution until neutral to obtain 2,3,5-tris Chloro-4-fluoronitrobenzene, yield 89%.
[0027] 24.5 g (0.1 mol) of 2,3,5-trichloro-4-fluoronitrobenzene, 11.7 g (0.2 mol) of anhydrous potassium fluoride, and 2 g of tetrabutylammonium bromide in 60 mL of sulfolane at 160 °C After reacting for 12 h, 19.5 g of 2,4...
Embodiment 2
[0030] Take 43.5 g of the distillation residue of 2,4-dichloro-3-fluoronitrobenzene production process (from an enterprise in Quzhou, Zhejiang Province to prepare 2,4-dichloro-3-fluoronitrobenzene by nitration of 2,6-dichlorofluorobenzene Distillation residue with batch number HJ110837 in the benzene production process in 2011, of which 3,5-dichloro-4-fluoronitrobenzene content is 85.5%, 2,4-dichloro-3-fluoronitrobenzene content is 11.3%), Stir 1.5 g of iron powder and 60 mL of concentrated sulfuric acid to raise the temperature to 150 °C, start chlorine gas flow, react for 8 h, stop chlorine flow, cool down, pour the reaction solution into ice water, wash the organic phase with NaOH aqueous solution until neutral to obtain 2,3, 5-trichloro-4-fluoronitrobenzene, yield 86.5%.
[0031] 24.5 g (0.1 mol) of 2,3,5-trichloro-4-fluoronitrobenzene, 11.7 g (0.2 mol) of anhydrous potassium fluoride, and 60 mL of tetramethylurea were reacted at 180 °C for 10 h, and distilled under reduce...
Embodiment 3
[0034] Take 44.0 g of the distillation residue of the production process of 2,4-dichloro-3-fluoronitrobenzene (from an enterprise in Linhai, Zhejiang to prepare 2,4-dichloro-3-fluoronitrobenzene by nitration of 2,6-dichlorofluorobenzene Distillation residue with batch number YT20110622 in the benzene production process in 2011, in which 3,5-dichloro-4-fluoronitrobenzene content is 80.9%, 2,4-dichloro-3-fluoronitrobenzene content is 14.3%) and 80 mL of chlorosulfonic acid was warmed up to 120 °C and started to pass chlorine gas, reacted for 10 h, stopped the chlorine flow, poured the reaction solution into a large amount of ice water, let it stand, and filtered to obtain 2,3,5-trichloro-4-fluoronitrobenzene, The yield is 90%.
[0035] 24.5 g (0.1 mol) of 2,3,5-trichloro-4-fluoronitrobenzene, 11.7 g (0.2 mol) of anhydrous potassium fluoride, 2 g of polyethylene glycol-6000 and 60 mL of benzonitrile were heated to Reacted at 150 °C for 12 h, and distilled under reduced pressure ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com