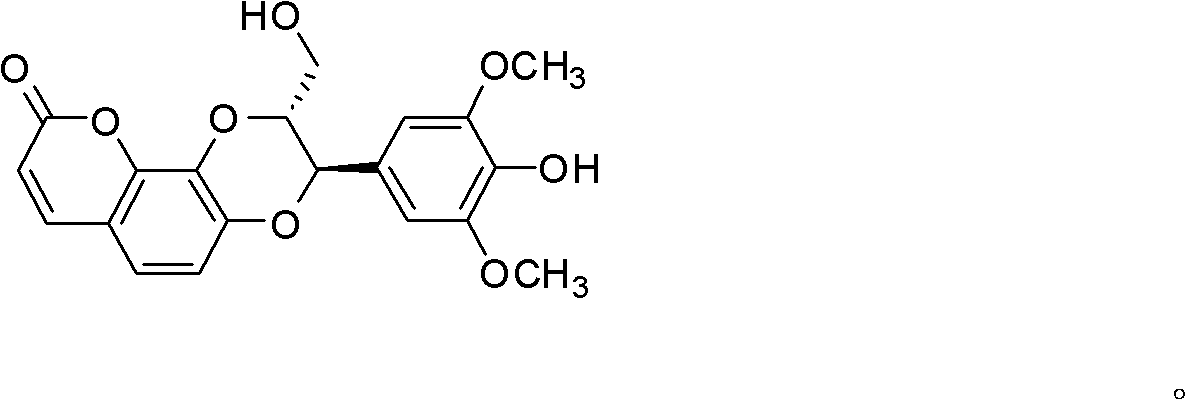
Application of isodaphnetin compound in preparation of antidiabetic medicines
An anti-diabetic technology of daphnein, which is applied in the fields of active ingredients of heterocyclic compounds, drug combinations, metabolic diseases, etc., can solve the problems such as the anti-diabetic effect of Phnom Penh daphne, which has not yet been seen, and achieve the protection and repair of islet cells, good resistance Diabetes drug efficacy, the effect of large clinical application value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] Preparation of Isorphinoid
[0017] 7.5kg Phnom Penh daphne medicinal material (commercially available) is crushed and extracted by percolation with 150L 75% ethanol, and the extract is concentrated under reduced pressure to obtain 8L extract. Ethyl extraction, collecting the ethyl acetate part, weighing 125g, the ethyl acetate part was subjected to silica gel H (200-300 mesh) column chromatography twice, chloroform: acetone (10:1-1:1), chloroform: methanol (50:1~1:1) gradient elution, SephadexLH-20 column chromatography and reversed-phase silica gel (Merck), isolated and obtained 108 mg of the compound isorphinoid.
[0018] The isoretasin prepared in Example 1 was used in the following experiments.
Embodiment 2
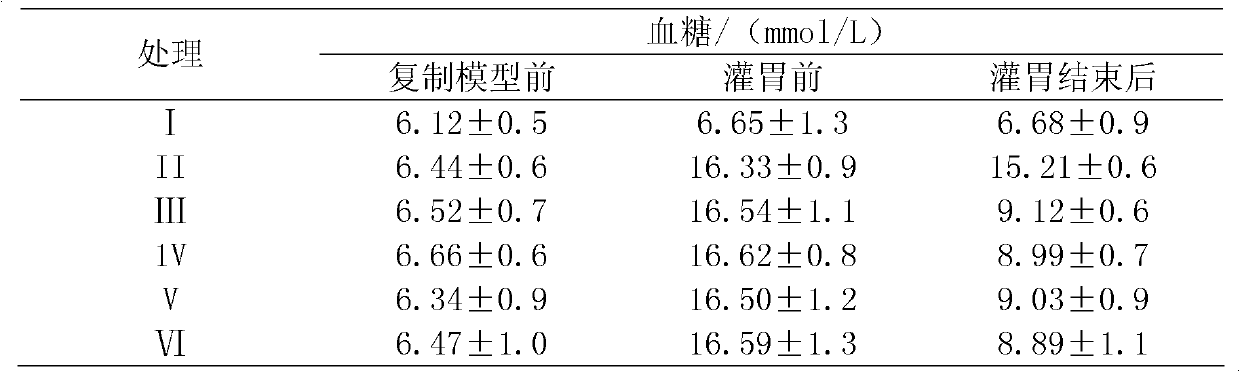
[0020] Effects of Isoretelin on Serum Insulin and Glucagon in Rats
[0021] experimental method
[0022] In this experiment, a high-sugar and high-fat diet combined with low-dose streptozotocin (STZ) was used as a rat model of type 2 diabetes. This model is more in line with the pathogenesis of ordinary type 2 diabetes in humans and is conducive to the discovery of therapeutic drugs for type 2 diabetes. Male SD rats were fed high-sugar and high-fat diet for 1 month to induce insulin resistance, and were given a small dose of streptozotocin (STZ) 25 mg / kg and injected intraperitoneally to establish a type 2 diabetic rat model. Drugs were then injected intraperitoneally, and the blood glucose, serum insulin, glucagon, mental state, body weight, water intake, food intake, and urine of the mice were used as detection indicators (Pharmacological Research. 2005, 52: 313-320).
[0023] Experimental steps:
[0024] 1. Material Isorphinoid is prepared from Example 1
[0025] 1.1 Exp...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com