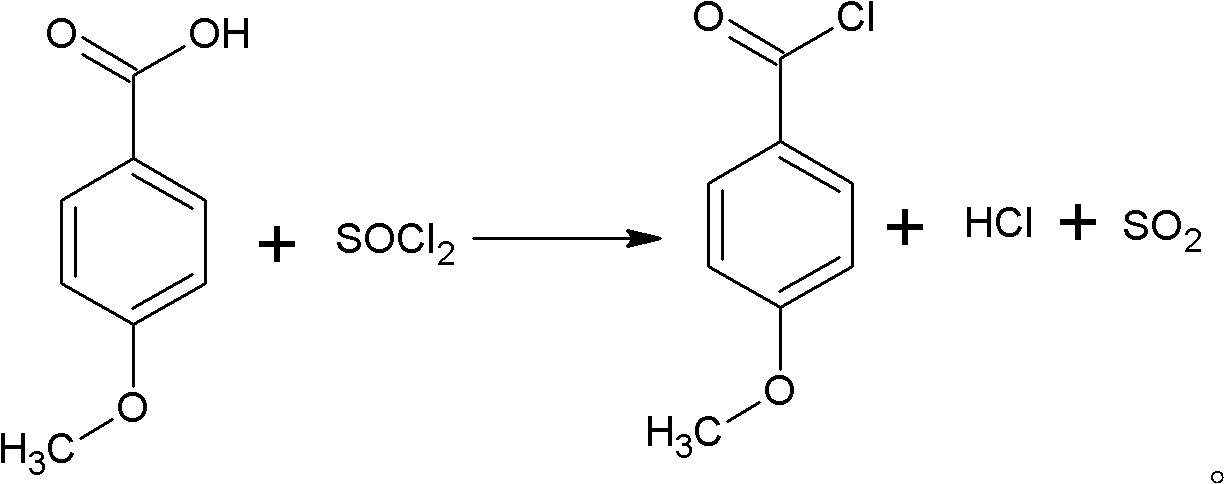
Preparation method of p-anisoyl chloride
A technology of methoxybenzoyl chloride and methoxybenzoic acid, which is applied in the field of preparation of p-methoxybenzoyl chloride, can solve the problems of environmental pollution, toxicity, and high requirements for use and transportation, and achieves reduction of environmental pollution, reduction of The effect of loss
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
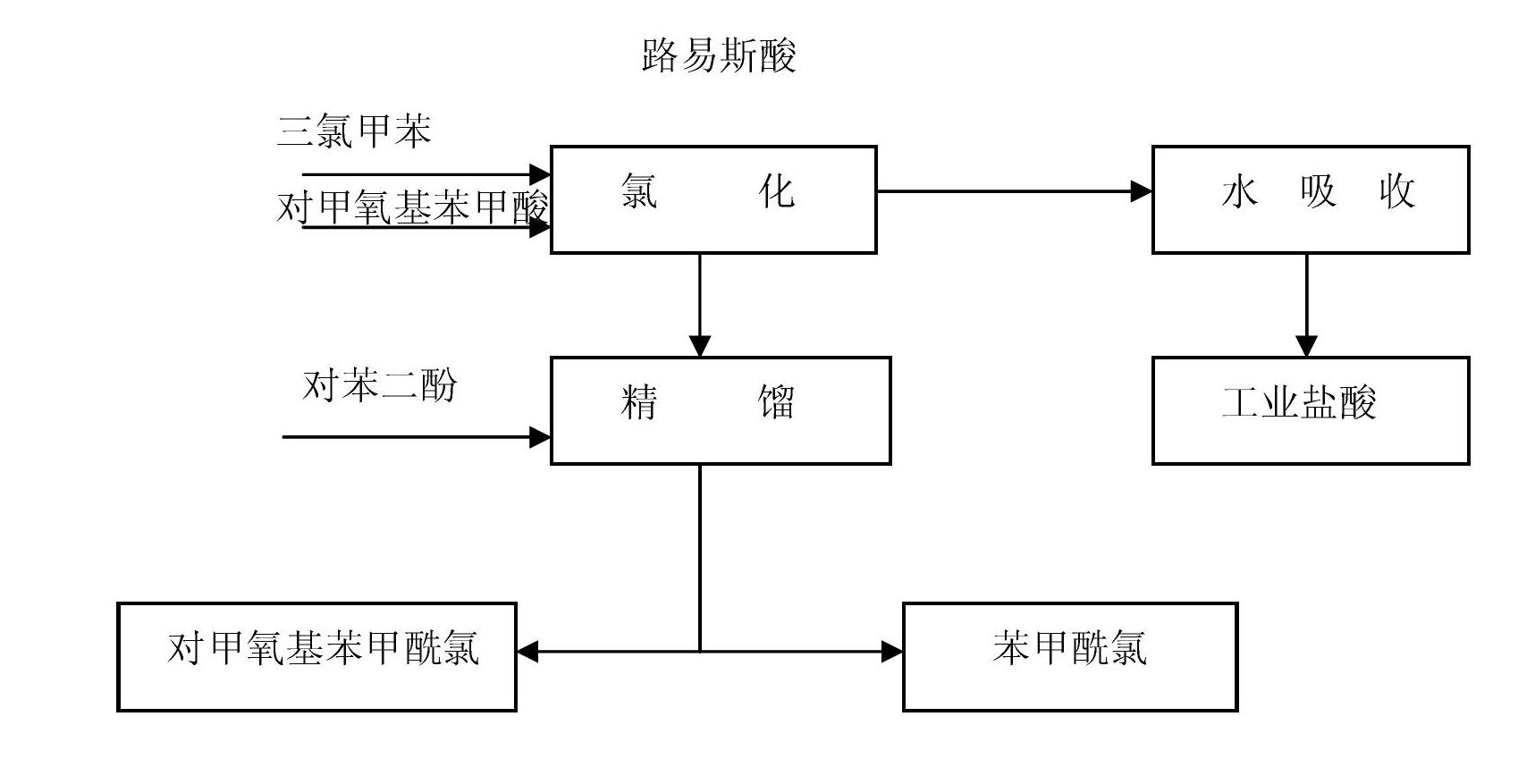
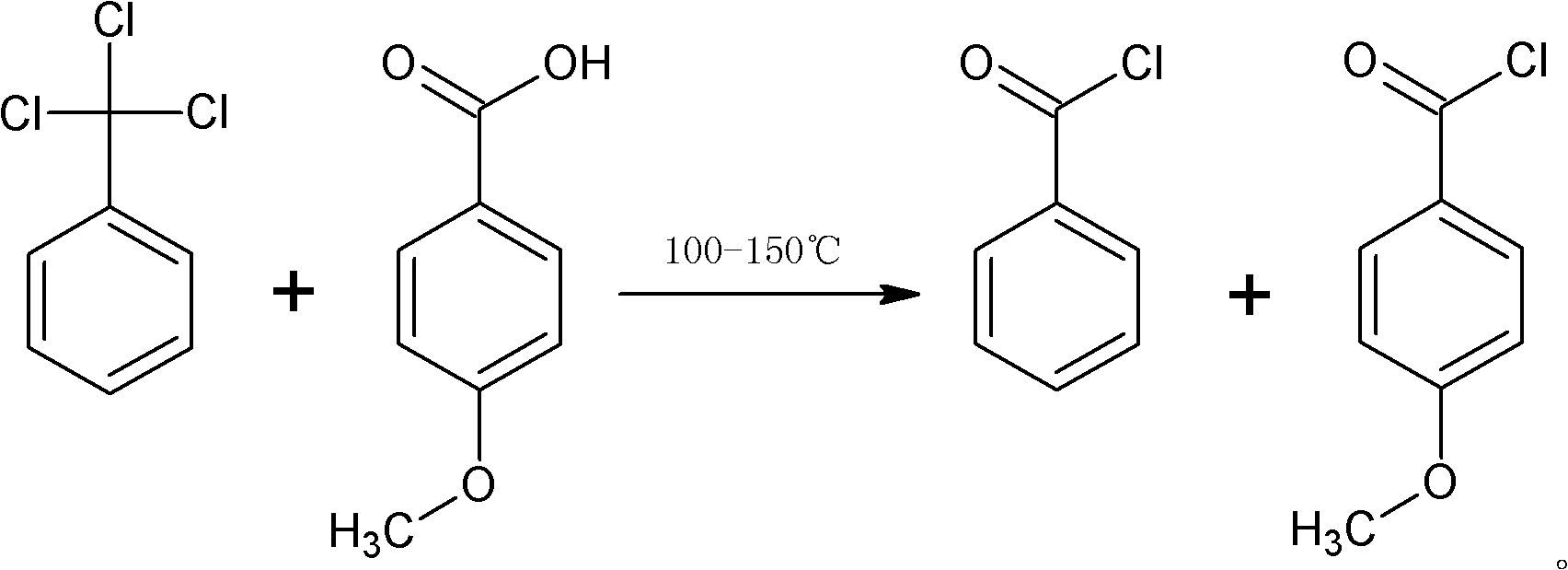
[0020] The p-methoxybenzoyl chloride preparation method of the present embodiment is as figure 1 As shown, the specific process is:
[0021] 1) Add 1 mol trichlorotoluene and 0.5 g of zinc chloride to the reaction bottle, open the stirrer inserted into the reaction bottle, add 1 mol p-methoxybenzoic acid, heat up to 150 ° C in 1 hour, keep the temperature for 5 hours, and react The tail gas in the water is absorbed into 30% hydrochloric acid;
[0022] 2) After the reaction, cool the temperature of the reaction material in the reaction bottle to 25°C, add 1g of hydroquinone, transfer the reaction material in the reaction bottle to the rectification column for vacuum distillation (the rectification column adopts Φ24* 1500, glass filler); finally obtain p-methoxybenzoyl chloride 165g, obtain benzoyl chloride 133g simultaneously.
[0023] The content of p-methoxybenzoyl chloride is 99.5%, the content of benzoyl chloride is 99.7%, the yield of benzoyl chloride is 95%, and the yie...
Embodiment 2
[0025] The p-methoxybenzoyl chloride preparation method of the present embodiment is basically the same as embodiment one, and the difference is: 1. in the 1) step, replace zinc chloride 0.5g with ferric chloride 0.5g; 2. through 1 3. react for 6 hours; 4. in 2) step, the cooling temperature of material is 15 ℃; 5. finally obtain p-methoxybenzoyl chloride 163g, obtain benzoyl chloride 131g simultaneously.
[0026] The content of benzoyl chloride was 99.7%, the content of p-methoxybenzoyl chloride was 99.5%, the yield of benzoyl chloride was 95%, and the yield of p-methoxybenzoyl chloride was 97% by gas chromatography AG6820.
Embodiment 3
[0028] The concrete process of the p-methoxybenzoyl chloride preparation method of the present embodiment is:
[0029] 1) Add 600kg of trichlorotoluene and 456kg of p-methoxybenzoic acid into the glass-lined reactor, add 0.5kg of Lewis acid catalyst at the same time, stir, first raise the temperature to 80 degrees for 1 hour, then raise the temperature to 130 degrees for 1 hour , and finally raised the temperature to 150°C, and reacted at a constant temperature for 10 hours, and the tail gas in the reaction was absorbed by water to become 30% hydrochloric acid;
[0030] 2) the temperature of the reaction material in the reactor is cooled to 20°C, and is transferred to a 15-meter high rectification tower (the rectification tower adopts an enamel corrugated packing tower), and 0.5kg of hydroquinone is added, and rectification under reduced pressure is carried out to obtain the Methoxybenzoyl chloride 496kg and benzoyl chloride 409kg.
[0031] The content of benzoyl chloride was...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com