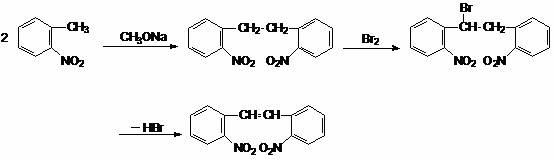
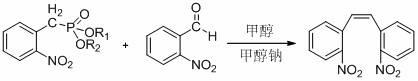
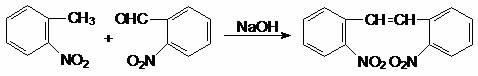
Synthesis method for 2,2'-dinitryl diphenylethene
A technology of dinitrostilbene and synthesis methods, which is applied in chemical instruments and methods, preparation of organic compounds, organic chemistry, etc., can solve the problems of cumbersome steps, lack of supply of o-nitrophosphonate, etc., and achieve simple equipment , the reaction conditions are easy to control, and the process flow is short
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Add 2.8g (0.02mol) of o-nitrotoluene, 3.1g (0.02mol) of o-nitrobenzaldehyde, and 0.5g (0.00155mol) of tetrabutylammonium bromide into a 100mL four-neck flask, then add 15mL of toluene and stir. And keep the temperature at 30~35°C, add dropwise 1.2g of 50% NaOH solution (containing 0.6g, 0.015molNaOH) for 1 hour, after the dropwise addition, add 0.2g (0.005mol) of solid sodium hydroxide, and continue the reaction for 5 ~12 hours. The reaction solution was washed with water to neutrality, the organic layer was evaporated toluene solvent under reduced pressure, and finally an appropriate amount of methanol solution was added to precipitate a solid product, which was filtered and dried to obtain 4.3 g of yellow solid 2,2'-dinitrostilbene, yield It is 79.6%, and the purity is more than 97%.
[0047] 1 HNMR (400MHz, CDCl 3 ): 7.46~7.50 (t, 2H), 7.57 (s, 2H), 7.65~7.69(t, 2H), 7.81~7.85(d, 2H), 8.04~8.06 (d, 2H).
[0048]
Embodiment 2
[0050] Add 2.8g (0.02mol) of o-nitrotoluene, 3.1g (0.02mol) of o-nitrobenzaldehyde, and 0.9g (0.0028mol) of tetrabutylammonium bromide into a 100mL four-neck flask, then add 15mL of toluene and stir. And keep the temperature at 30~35℃, add dropwise 1.2g (containing 0.6g, 0.015molNaOH) of 50% NaOH solution for 1h, after the dropwise addition, add 0.4g (0.01mol) of solid sodium hydroxide, and continue the reaction for 5 ~12 hours. The reaction solution was washed with water to neutrality, the organic layer was evaporated toluene under reduced pressure, and finally an appropriate amount of methanol solution was added to precipitate a solid product, which was filtered and dried to obtain 4.4 g of yellow solid 2,2'-dinitrostilbene, yield It is 81.4%, and the purity is more than 98%.
[0051] 1 HNMR (400MHz, CDCl 3 ): 7.46~7.50 (t, 2H), 7.57 (s, 2H), 7.65~7.69(t, 2H), 7.81~7.85(d, 2H), 8.04~8.06 (d, 2H).
[0052]
Embodiment 3
[0054]Add 2.8g (0.02mol) of o-nitrotoluene, 3.1g (0.02mol) of o-nitrobenzaldehyde, and 0.5g (0.00155mol) of tetrabutylammonium bromide into a 100mL four-neck flask, then add 15mL of benzene and stir. And keep the temperature at 30~35°C, add dropwise 1.2g of 50% NaOH solution (containing 0.6g, 0.015molNaOH) for 1h, after the dropwise addition, add 0.2g (0.005mol) of solid sodium hydroxide, and continue the reaction for 5 ~12 hours. The reaction solution was washed with water to neutrality, the organic layer was evaporated with benzene solvent under reduced pressure, and finally an appropriate amount of methanol solution was added to precipitate a solid product, which was filtered, dried, and dried to obtain 4.3 g of yellow solid 2,2'-dinitrostilbene. The rate was 79.6%, and the purity was >96%.
[0055] 1 HNMR (400MHz, CDCl 3 ): 7.46~7.50 (t, 2H), 7.57 (s, 2H), 7.65~7.69(t, 2H), 7.81~7.85(d, 2H), 8.04~8.06 (d, 2H).
[0056]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com