Enzymatic synthesis method of chiral beta-hydroxyl ester compound
A synthetic method and compound technology, applied in the field of enzyme-catalyzed synthesis of chiral β-hydroxy ester compounds, can solve the problems of high production cost, impractical and economical, and expensive chiral reagents of β-hydroxy esters, and achieve high practicality The effect of value, low production cost and high conversion rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
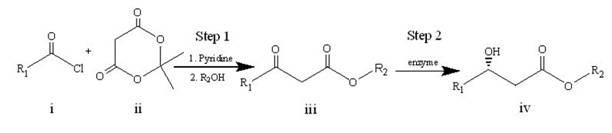
[0017] A specific embodiment of the present invention is to prepare an intermediate (S)-3-hydroxytetradecanoic acid methyl ester for the synthesis of orlistat by an enzyme-catalyzed synthesis method, and the specific steps are as follows:
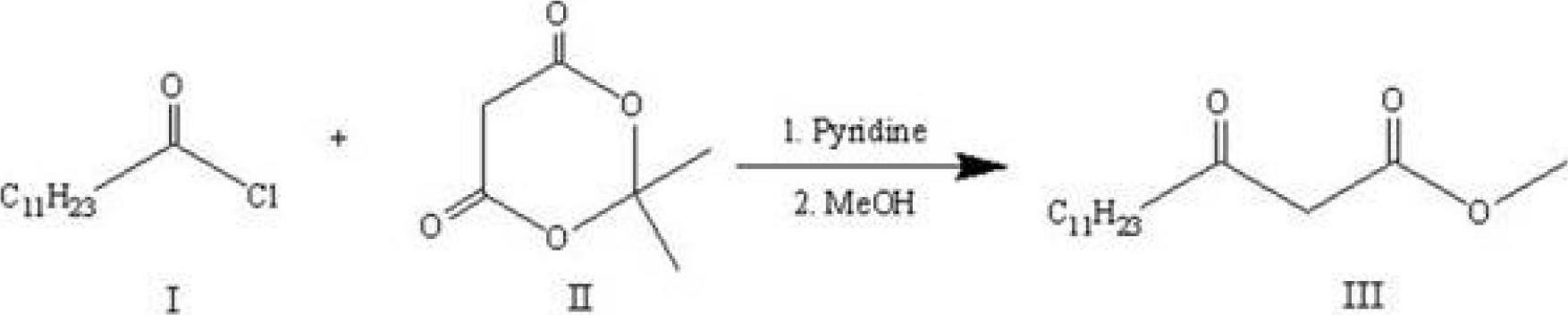
[0018] 1) Let lauroyl chloride Ⅰ and cycloisopropylidene malonate Ⅱ react for about 2 hours under the conditions of 0°C to room temperature, pyridine as an acid-binding agent, and dichloromethane as a solvent. The obtained product is extracted and concentrated, and then added Reflux in methanol for 4-6 hours, and then recrystallize to obtain methyl lauroyl acetate III. The synthetic route is as follows:
[0019]
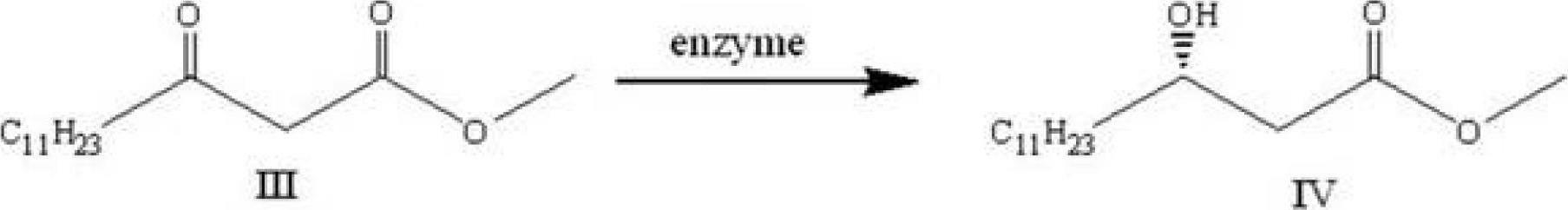
[0020] 2) Add the methyl lauroyl acetate III, ketoreductase and coenzyme obtained in step 1) into the phosphate buffer solution with pH=6~8, and react at 15~40°C for 2~24 hours to obtain optically pure ( S)-Methyl 3-hydroxytetradecanoate IV. Among them, the amount of ketoreductase added is 1~50g / L, the amount of methyl lauroy...
Embodiment 1
[0026] 1) Preparation of methyl lauroyl acetate:
[0027] Under nitrogen protection and stirring in an ice bath at 0°C, 60 mL of dichloromethane, pyridine (9 mL, 0.1112 mol) and cycloisopropylidene malonate (8 g, 0.0556 mol) were added to a 250 mL reaction flask. Lauroyl chloride (13.6 mL, 0.059 mol) was slowly added dropwise to the reaction solution, and the reaction mixture was stirred in an ice bath at 0°C for 10 min, and the reaction solution was slowly raised to room temperature, and continued to stir for 2 h. After the reaction was completed, the reaction mixture was washed successively with hydrochloric acid (1M), water, and saturated brine, and the organic phase was dried over anhydrous sodium sulfate, filtered, and concentrated.
[0028] Dissolve the concentrate in methanol (50 mL), heat the reaction solution under reflux for 5 hours, then slowly lower the reaction solution to room temperature, and then recrystallize at a low temperature of -30°C to obtain a lig...
Embodiment 2
[0032] 1) Preparation of methyl lauroyl acetate:
[0033] With embodiment 1.
[0034] ) Preparation of (S)-3-hydroxy tetradecanoic acid methyl ester:
[0035] Add 9.4g methyl lauroyl acetate, 0.6L dimethyl sulfoxide, 6L phosphate buffer solution (Na 2 HPO 4 / NaH 2 PO 4 , 0.1M, pH=7.0), 60g ketoreductase ADH-RE, 20g glucose, 65g glucose dehydrogenase and 0.05g NADP, stirred in a water bath at 35℃, reacted for 12h to obtain optically pure (S)-3- Methyl tetradecanoate. Samples were taken for GC analysis, and the conversion rate was >90%; HPLC analysis was performed, and the ee value was greater than >95%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com