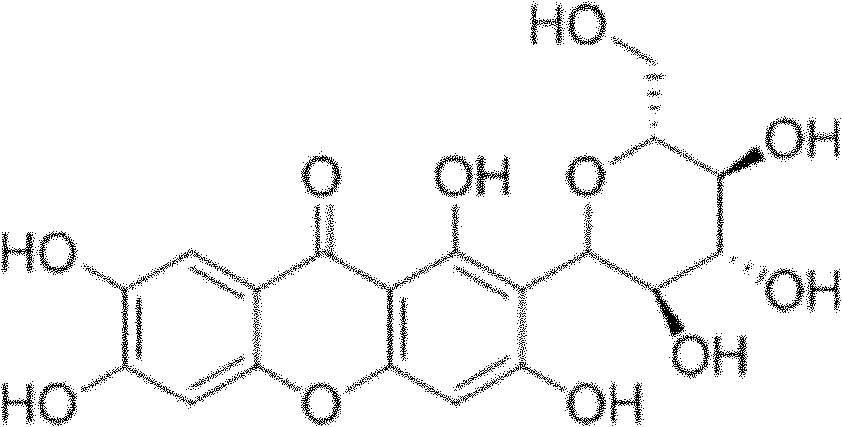
Composition with blood uric acid adjusting function
A composition and blood uric acid technology, applied in the field of medicine, can solve the problems of renal function impact, uric acid rebound, and ineffective effects, etc., and achieve the effect of reducing the impact of renal function, promoting uric acid excretion, and outstanding health care effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
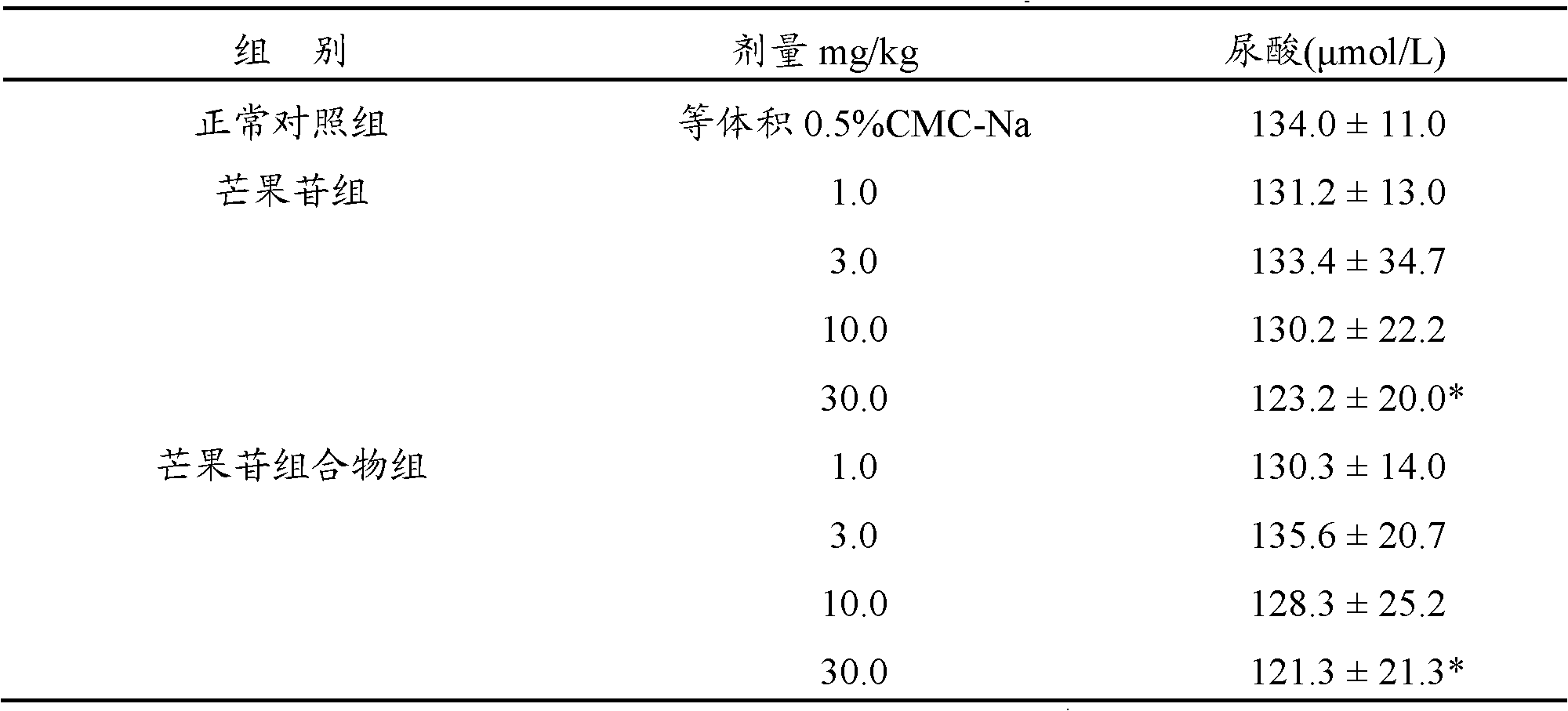
[0026] Example 1: Effect of the mangiferin composition of the present invention on uric acid in normal mice
[0027] 110 male Kunming mice, weighing 18-22 g, were randomly divided into 9 groups, 10 in each group, respectively: normal control group, 4 different dosage groups of mangiferin, and the composition 4 of mangiferin: Poria cocos=1:20 different dosage groups (hereinafter referred to as the mangiferin composition group). The test compound was prepared into a suspension with 0.5% sodium carboxymethylcellulose (0.5% CMC-Na). The normal control group was given an equal volume of vehicle (0.5% CMC-Na) by intragastric administration, and the mangiferin group and the mangiferin composition group were intragastrically administered according to the mangiferin content of 1.0, 3.0, 10.0, and 30.0 mg / kg, and 10ml / kg was administered intragastrically every day Administration 2 times, 5 consecutive times. One hour after the last administration, the eyeball was removed to take blood...
Embodiment 2
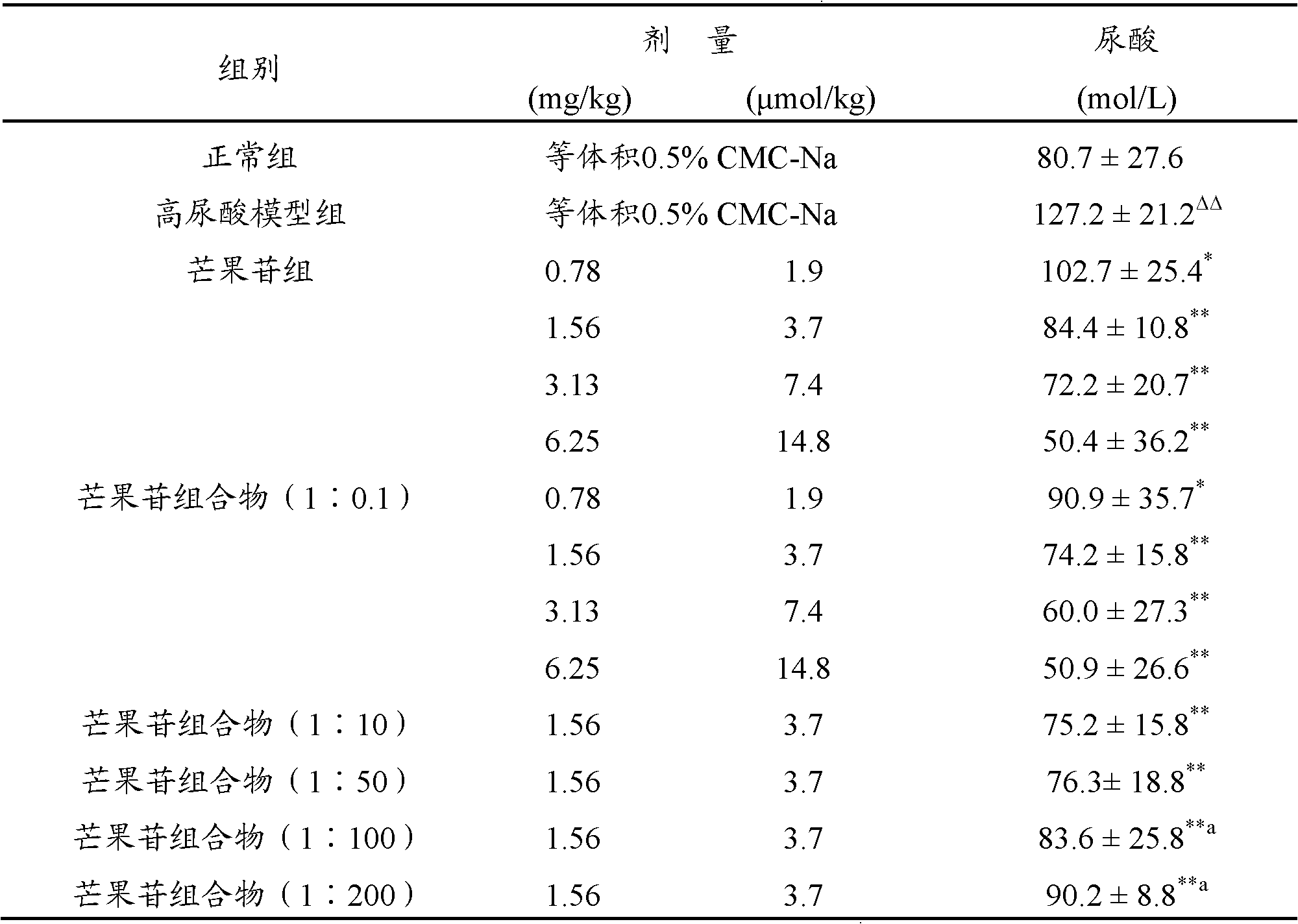
[0032] Example 2: Effect of the mangiferin composition of the present invention on serum uric acid in mice with hyperuricemia
[0033]140 healthy male Kunming mice, weighing 18-22 g, were provided by the Experimental Animal Center of Kunming Medical College [Experimental animal production license number: SCXK (Dian) 2005-2008]. Animals were randomly divided into normal control group, hyperuricemia model group, mangiferin: Poria cocos=1:0.1 composition (calculated according to mangiferin content) 0.78, 1.56, 3.13 and 6.25 mg / kg dosage groups and mangiferin: Poria cocos different Proportional dose group. The test compound was formulated into a suspension with 0.5% sodium carboxymethylcellulose (0.5% CMC-Na), and administered by intragastric administration, twice a day for five consecutive times.
[0034] Modeling method of hyperuricemia: mice were intraperitoneally injected with 400 mg / kg oxonic acid potassium salt 2 hours before blood sampling to inhibit uricase activity and c...
Embodiment 3
[0039] Example 3: Effect of the mangiferin composition of the present invention on the excretion of phenol red in normal rats
[0040] 70 male SD rats, weighing 130-150g, were randomly divided into 7 groups, 10 in each group, respectively: normal control group, 3 different dose groups of mangiferin dose group, and the combination of mangiferin:poria cocos=1:30 3 different dosage groups of the compound were administered intragastrically with equal volume of vehicle (0.5% CMC-Na), mangiferin and mangiferin composition (calculated according to mangiferin content) 3.0, 6.0, 12.0mg / kg, and 10ml / kg per day Dosing once, 7 times in total, refer to the literature (H.G Vogel, W.H Vogel, edited. Du Guanhua, Li Xuejun, Zhang Yongxiang, et al. Translated. Pharmacological Experiment Guide--New Drug Discovery and Pharmacological Evaluation. First Edition, 2001, p238-239.) method: 3% phenol red (2.5ml / kg) was injected into the tail vein 30 minutes after the last administration, and 20 μL of b...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com