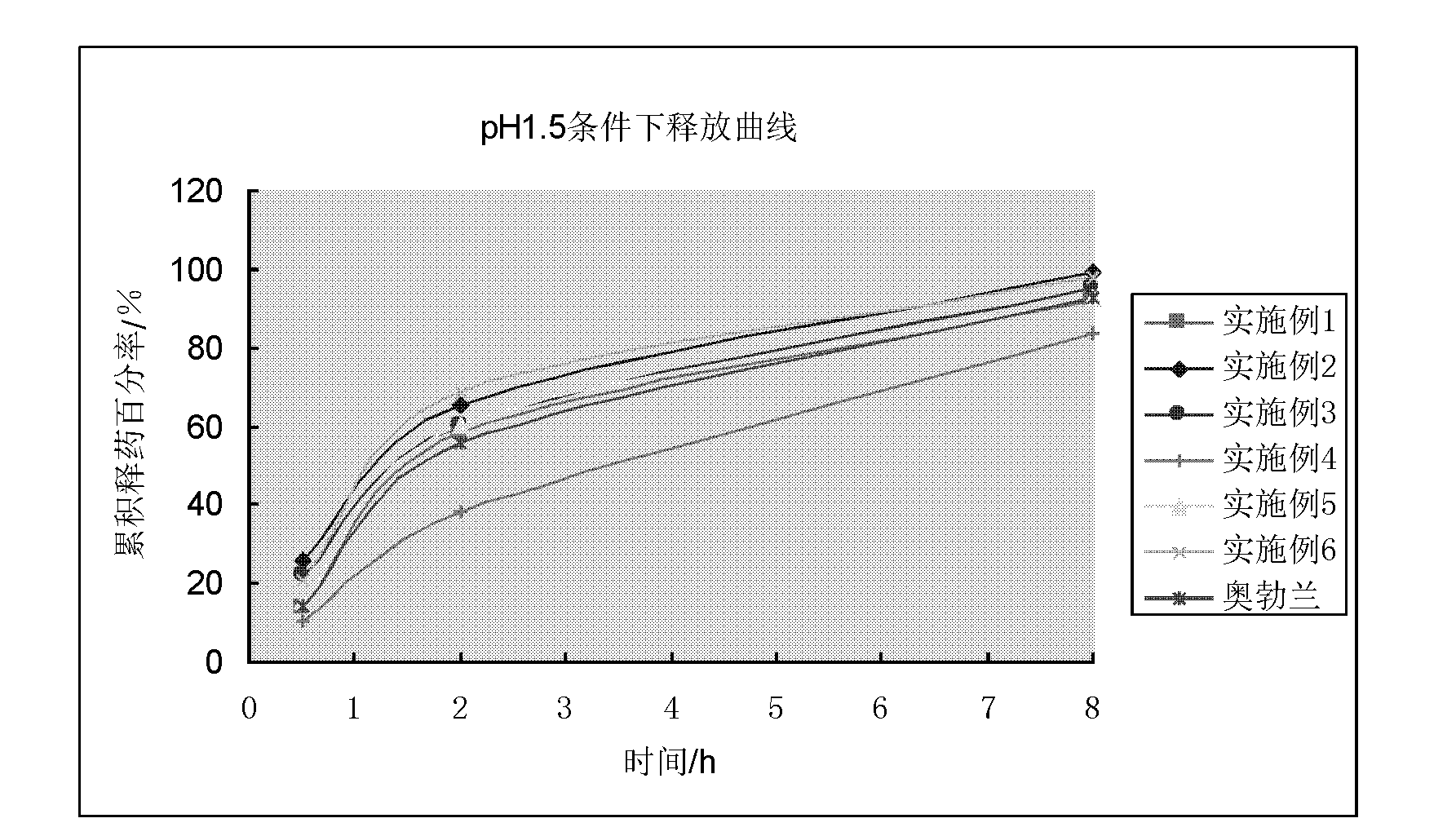
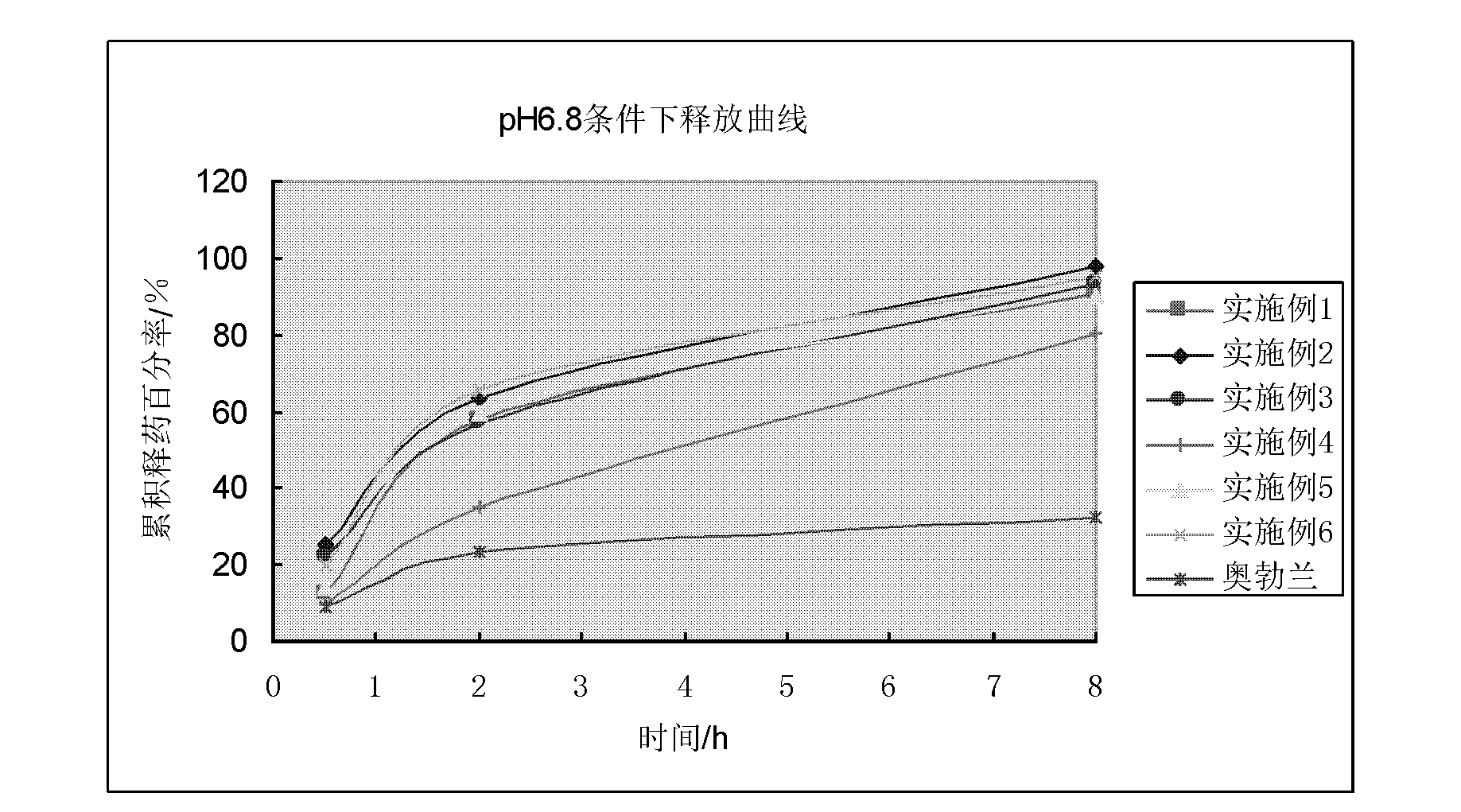
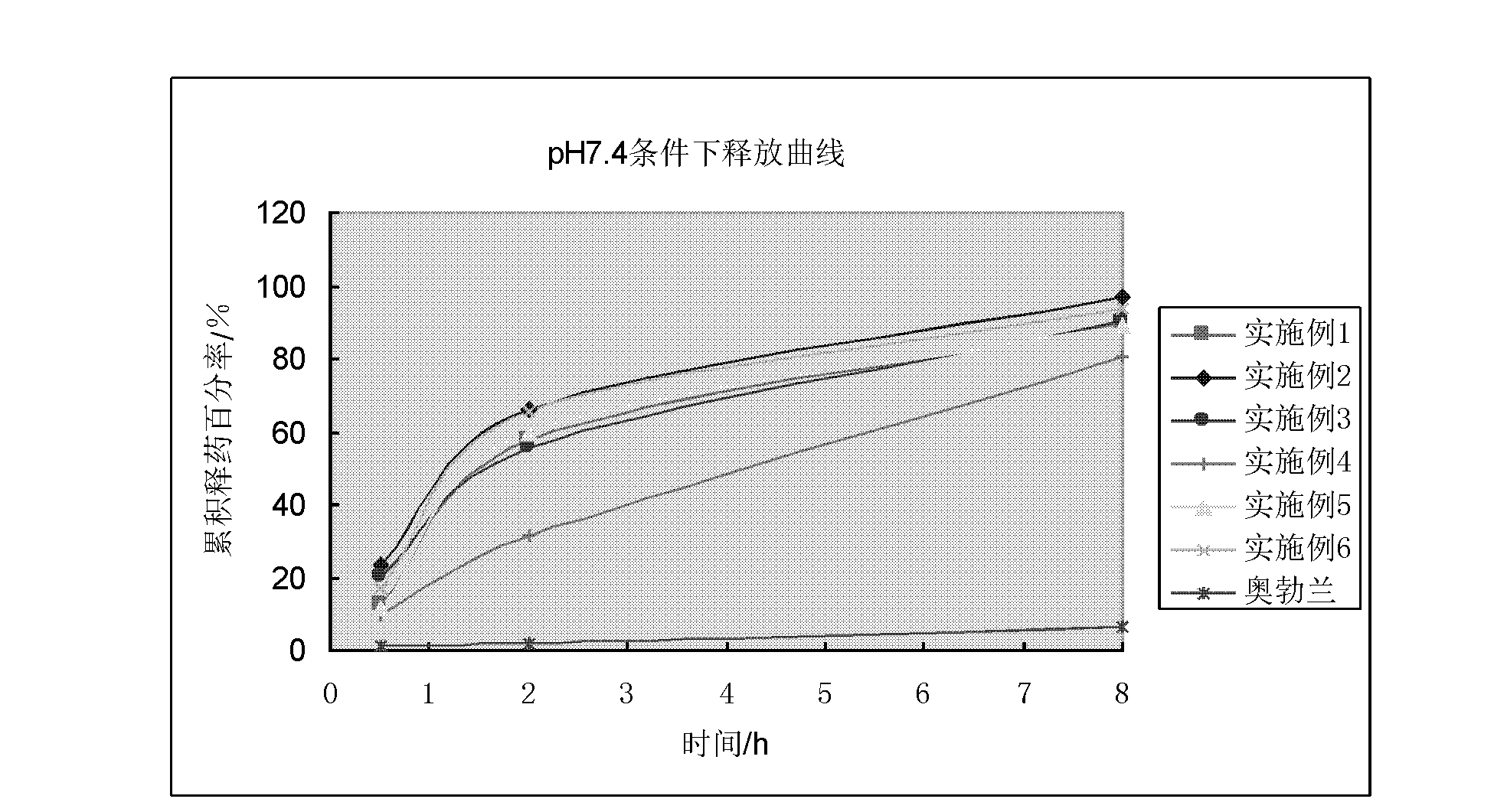
Vincamine sustained-release pellet preparation and preparation method thereof
A technology of slow-release pellets and vincamine, which is applied in the direction of pharmaceutical formulations, medical preparations containing active ingredients, and devices that make drugs into special physical or taking forms, and can solve the problems of intestinal absorption and drug utilization. Not high, unfavorable for the release of O'Brien, etc., to achieve the effect of solving pH value dependence, convenient drug use, and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Prescription ratio of vincamine sustained-release pellet preparation:
[0041]
[0042] Preparation:
[0043] (1) Material preparation:
[0044] a The main drug vincamine is micronized, with an average particle size range of 5 μm;
[0045] B, add 60g ethanol in povidone, be mixed with solution, add vincamine, make the solution containing medicine, standby;
[0046] c, add 140g water in tartaric acid, be mixed with organic acid solution, set aside;
[0047] (2) Preparation of acidic blank cores: get blank cores and put them in a fluidized bed, spray the organic acid solution obtained by c on the blank cores to make acidic blank cores;
[0048] (3) Prepare drug-loaded pellet cores: take acidic blank pellet cores and put them in a fluidized bed, spray the drug-containing solution obtained in b on the acidic blank pellet cores to make drug-loaded pellet cores;
[0049] (4) Prepare slow-release drug-loaded pellets: take the drug-loaded pellet core and put it in a flui...
Embodiment 2
[0053] Prescription ratio of vincamine sustained-release pellet preparation:
[0054]
[0055] Preparation:
[0056] (1) Material preparation:
[0057] a The main drug vincamine is micronized, with an average particle size range of 20 μm;
[0058] B, add 60g ethanol, 100g water in copovidone, be mixed with solution, add vincamine, make the solution containing medicine, standby;
[0059] c. Add 40g of water to the citric acid to prepare an organic acid solution for subsequent use;
[0060] (2) Prepare acidic blank cores: get blank cores and put them in a coating pan, spray the organic acid solution obtained in c on blank cores to make acidic blank cores;
[0061] (3) Preparation of drug-loaded pellet cores: take acidic blank pellet cores and put them in a coating pan, spray the drug-containing solution obtained in b on the acidic blank pellet cores to make drug-loaded pellet cores;
[0062] (4) Prepare slow-release drug-loaded pellets: take the drug-loaded pellet core and ...
Embodiment 3
[0066] Prescription ratio of vincamine sustained-release pellet preparation:
[0067]
[0068] Preparation:
[0069] (1) Material preparation:
[0070] a The main drug vincamine is micronized, with an average particle size range of 15 μm;
[0071] B, add 380g water in hydroxypropyl methylcellulose, starch, be mixed with solution, add vincamine, make the solution containing medicine, standby;
[0072] c. Add 120g of water to tartaric acid and malic acid to prepare an organic acid solution for subsequent use;
[0073] (2) Preparation of acidic blank core: take the blank core and put it in extrusion spheronization, spray the organic acid solution obtained by c on the blank core to make an acidic blank core;
[0074] (3) Prepare drug-loaded pellet cores: take acidic blank pellet cores and place them in extrusion spheronization, spray the drug-containing solution obtained in b on the acidic blank pellet cores to make drug-loaded pellet cores;
[0075] (4) Preparation of slow...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com