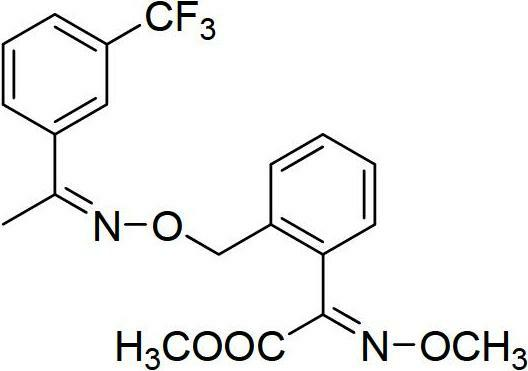
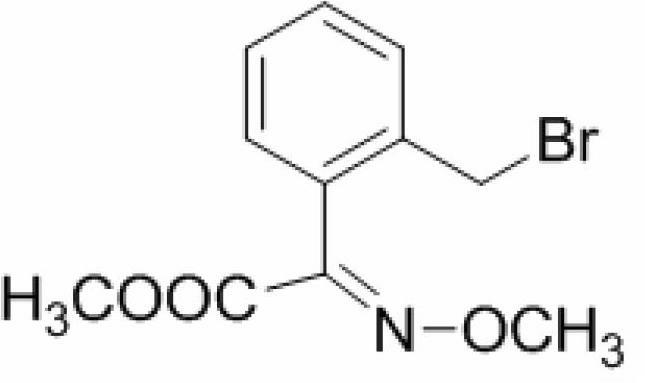
Method for synthesizing trifloxystrobin
A synthetic method, the technology of trifloxystrobin, which is applied in the field of preparation of fine chemical products, can solve the problems of unstable chemical properties, difficult processing, high purity requirements, etc., and achieve the reduction of production cost and risk factor, simple and practical preparation method, and high product quality. The effect of high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] A kind of synthetic method of trifloxystrobin, the steps are as follows:
[0029] 1) In a 1L four-neck flask equipped with a reflux and mechanical stirring device, add 13.5g (E)-2-(2-methylphenyl)-2-methoxyiminoacetic acid methyl ester to 435ml tetrachloride In the mixed solution of carbon and 87ml of water, stir to dissolve the raw materials completely, add 4.87g of sodium acetate, then stir at room temperature for 30 minutes;
[0030] 2) Add 15g of liquid bromine dropwise to the above system, the dropping rate of liquid bromine is 1 drop every 5 seconds, after the dropwise addition, react for 20 minutes under the lighting condition of 65W fluorescent light, then add the commercially available fluorescent powder anthracene (emission wavelength is 410nm) and continue to illuminate the fluorescent lamp selected for the reaction for 30 minutes, then stop the light, continue to stir the reaction at room temperature for 1 hour, wash the reaction solution with saturated aque...
Embodiment 2
[0037] A kind of synthetic method of trifloxystrobin, the steps are as follows:
[0038] 1) In a 1L four-neck flask equipped with a reflux and mechanical stirring device, add 18.6g (E)-2-(2-methylphenyl)-2-methoxyiminoacetic acid methyl ester to 480ml tetrachloride In the mixed solution of carbon and 98ml of water, stir to dissolve the raw materials completely, add 4.87g of sodium acetate, then stir at room temperature for 30 minutes;
[0039] 2) Add 15g of liquid bromine dropwise to the above system, and the dropping rate of liquid bromine is 1 drop every 5 seconds. After the dropwise addition, react for 20 minutes under the lighting condition of 80W fluorescent light, and then add commercially available pyrimidine anthrone (emission wavelength is 468nm) and continue to illuminate the fluorescent lamp selected for the reaction for 30 minutes, then stop the light, continue to stir the reaction at room temperature for 1 hour, wash the reaction solution with saturated aqueous so...
Embodiment 3
[0046] A kind of synthetic method of trifloxystrobin, the steps are as follows:
[0047] 1) In a 1L four-neck flask equipped with a reflux and mechanical stirring device, add 25.5g (E)-2-(2-methylphenyl)-2-methoxyiminoacetic acid methyl ester to 510ml tetrachloride In the mixed solution of carbon and 102ml of water, stir to dissolve the raw materials completely, add 4.87g of sodium acetate, then stir at room temperature for 30 minutes;
[0048] 2) Add 15g of liquid bromine dropwise to the above system, and the drop rate of liquid bromine is 1 drop every 5 seconds. After the dropwise addition, react under 85W fluorescent light for 20 minutes, and then add commercially available phenanthrene (emission wavelength is 330nm) And after the fluorescent lamp selected for the reaction continues to illuminate for 30 minutes, stop the illumination, and after continuing to stir and react at room temperature for 1 hour, wash the reaction solution with saturated aqueous sodium bicarbonate s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com