Novel spirobifluorene compound 2-methyl-7-benzimidazolyl spirobifluorene, and method and application thereof
A technology of benzimidazolylspiro and dimethylspirobifluorene, which is applied in the field of new spirobifluorene compounds and their preparation, and achieves the effects of wide detection range, high fluorescence intensity and high thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
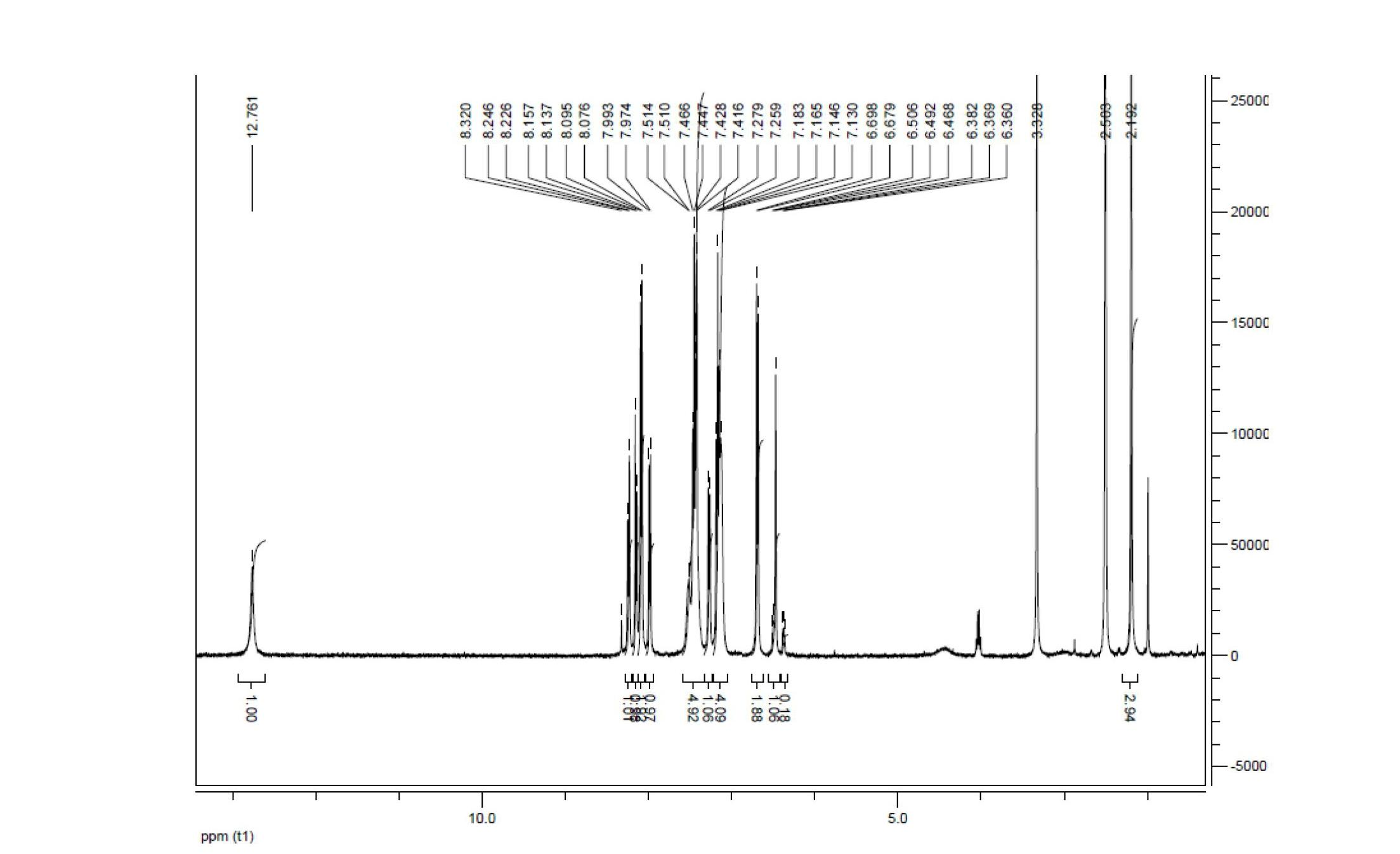
Embodiment 1
[0053] 1) Synthesis of 4,4'-dimethylbiphenyl
[0054] Under nitrogen protection, weigh 3g (125mmol) dry magnesium powder, 0.2g (1.2mmol) ferric chloride in a 100mL dry three-necked flask, then weigh 11g (64.3mmol) p-bromotoluene in 50mL and dry in a constant pressure dropping funnel. Inject 60 mL of anhydrous THF into the three-necked flask, and vigorously stir at room temperature. First quickly add 5% p-bromotoluene dropwise, and then slowly add dropwise after the reaction is triggered. After the dropwise addition was completed, the reaction was continued for 30 min, the reaction was stopped, cooled to room temperature, and filtered with suction to obtain a black filtrate, and the solvent was removed to obtain a black paste. Add 50mL of dichloromethane and 30mL of water in sequence, vibrate vigorously, and a large number of flocs appear in the solution. Slowly add dilute hydrochloric acid solution dropwise to the solution and shake continuously until the flocs disappear. ...
Embodiment 2
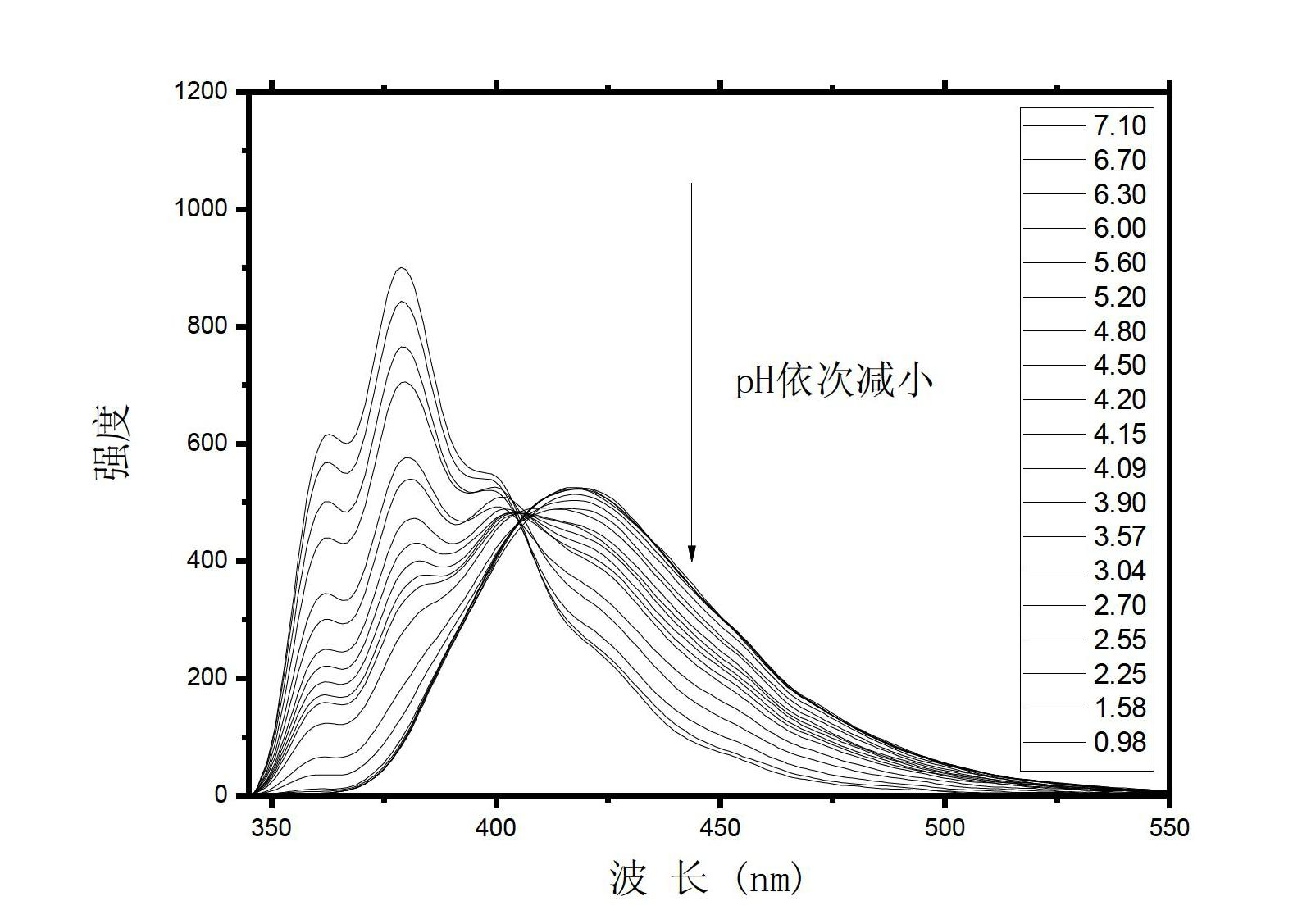
[0065] Example 2: Response of Compound Fluorescence Spectrum to pH
[0066] Compound (I) was formulated as 1 × 10 -5 M, the solvent is acetonitrile: water = 15:1, the pH is adjusted with different concentrations of NaOH and HCl solutions, and the fluorescence spectra at different pHs are tested. The test conditions are: the excitation wavelength is 338nm, the slit width is 5 / 5, and the voltage is 420V. Test results such as image 3 and Figure 4 .
[0067] from image 3 It can be clearly seen that the fluorescence emission peak of the compound red shifts continuously with the decrease of pH, and the maximum emission intensity also decreases continuously. The intensity of its maximum emission peak decreased from 900 to 530, and at the same time, the maximum emission peak was red-shifted from 380nm to 418nm, a red-shift of 38nm. The peak shape of the fluorescence spectrum also gradually changed from a more obvious triplet peak at neutral to a singlet peak. And throughout ...
Embodiment 3
[0069] Example 3: Response of Compound Absorption Spectrum to pH
[0070] Compound (I) was formulated as 1 × 10 -5 M, the solvent is acetonitrile: water = 15:1 (volume ratio), the pH is adjusted with different concentrations of NaOH and HCl solutions, and the absorption spectra at different pHs are tested. Test results such as Figure 5 and Image 6 .
[0071] from Figure 5 It can be seen that the influence of pH on the absorption spectrum of the compound is obvious. In a neutral environment, it can be clearly seen that it has two absorption spectra, which are 335nm and 352nm. When the environment becomes acidic, they red shift to 347nm and 365nm, respectively. Moreover, the second absorption peak becomes smoother, and two isotopes appear at 340nm and 352nm during the whole change process.
[0072] from Image 6 It can be seen that the influence of pH on the absorption spectrum of the compound is obvious. In a neutral environment, it can be clearly seen that it has t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| thermal decomposition temperature | aaaaa | aaaaa |
| decomposition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com