Fluorescent microsphere immunochromatographic test strip for detecting olaquindox residues and application thereof
A technology of immunochromatography test strips and fluorescent microspheres, which is applied in the direction of fluorescence/phosphorescence, material analysis through optical means, and measuring devices, can solve problems such as false positives, false negatives, and unsatisfactory sensitivity, and achieve high precision and Good sensitivity, dispersibility and stability, and the effect of improving analytical sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
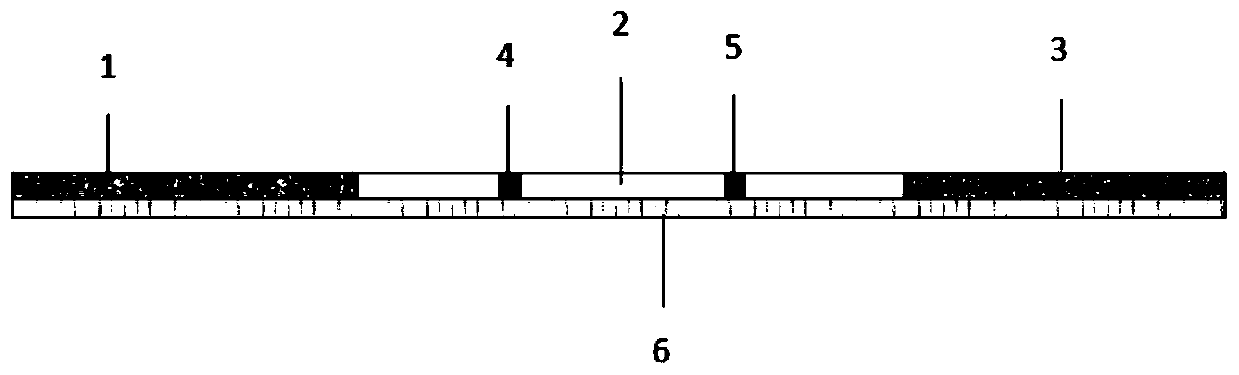
[0055] The preparation method of the time-resolved fluorescent microsphere immunochromatographic test strip for detecting residual olaquindox mainly comprises the following steps:
[0056] 1) Preparation of the sample binding pad 1: use the time-resolved fluorescent microspheres produced by Bangs Laboratories to label the anti-oquinethanol monoclonal antibody, and dilute it with a buffer system, soak the sample binding pad 1 in the dilution buffer, and Prepared after vacuum freeze-drying;
[0057] The fluorescent microsphere is a microsphere with a diameter of 100-300nm, which is coated with a polystyrene-coated fluorescent substance, and -COOH groups are connected on its surface, and the fluorescent substance is a europium complex.
[0058] 2) Preparation of nitrocellulose membrane 2: Spray the olaquindox hapten-carrier protein conjugate on the detection area range on the nitrocellulose membrane 2 to make detection area 4; spray goat anti-mouse secondary antibody onto the nit...
Embodiment 1
[0061] Synthesis of artificial antigen of olaquindox
[0062] 1) Synthesis of olaquindox hapten
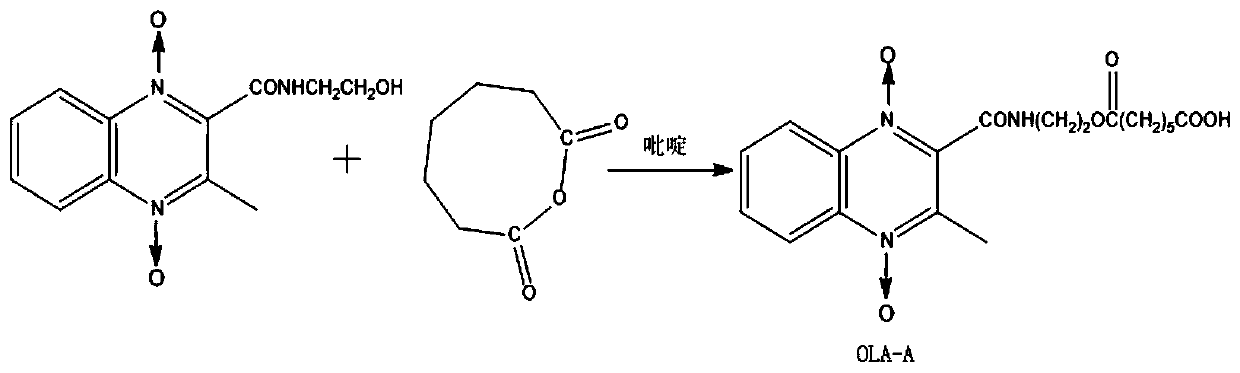
[0063] Accurately add 2.106g of olaquindox and 2.274g of oxocane-2,8-dione into a three-neck round-bottomed flask, add 85mL of pyridine, reflux at 115°C for 6h, then distill off pyridine under reduced pressure, add 60mL of ice-distilled water to the remaining mixture, 2mol L -1 Adjust the pH to 2.0-3.0 with HCl, and place it overnight at 4°C. Suction filtration under reduced pressure and washing with ice distilled water and drying, the obtained substance is the olaquindox hapten OLA-A, -A represents -CO(CH 2 ) 5 COOH; specific synthetic routes such as image 3 shown.
[0064] 2) Preparation of olaquindox-coated antigen and immunogen
[0065] Dissolve 0.04mmol OLA-A in 0.8mL N,N-dimethylformamide (DMF), add 0.04mmol N-hydroxysuccinimide (NHS) and 0.04mmol dicyclohexylcarbodiimide (DCC) , Stir at room temperature in the dark for 12 hours and then 2000r·min -1 Centrifuge for ...
Embodiment 2
[0086] Determination of antiserum titer:
[0087] The artificial antigens prepared in Example 1 and Comparative Example 1 were used to immunize BALB / C mice respectively. The artificial antigens were emulsified with complete Freund's adjuvant for the initial immunization. Booster immunization every day, a total of 3 booster immunizations, emulsified with incomplete adjuvant for booster immunization, immunological dose is 150 μg / mouse, after 14 days (days) after booster immunization, blood was collected from the tail of the mouse to measure the titer of multiple antiserum. The titer of antiserum was determined by ELISA method after doubling dilution with blocking solution, the mouse serum before immunization was used as negative control, and the OD of positive serum was used as 450nm Value and Negative Serum OD 450nm The dilution where the value ratio is greater than 2.1 is the antiserum titer, and the results are shown in Table 1. Finally, final immunization was carried out, ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com