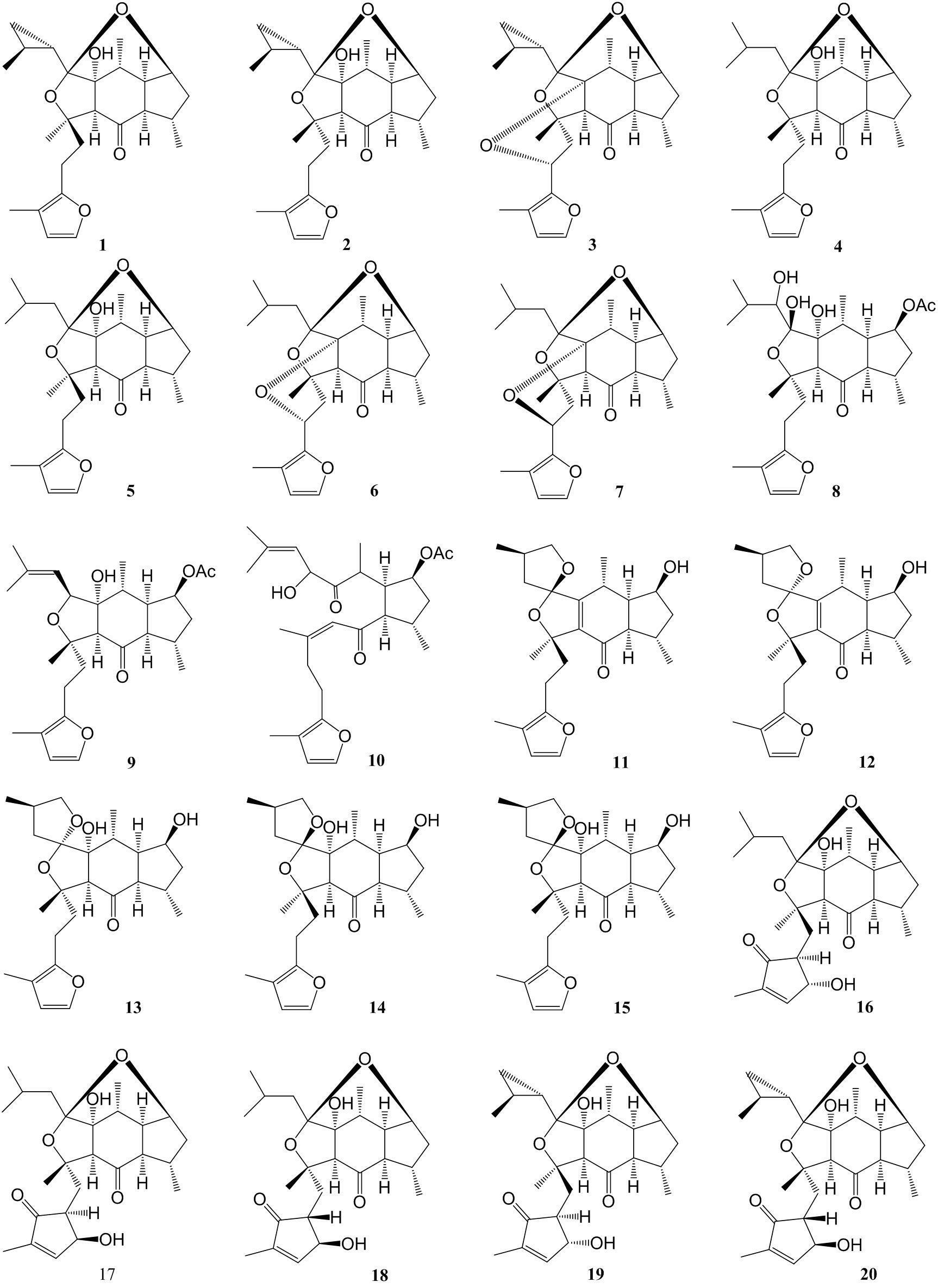
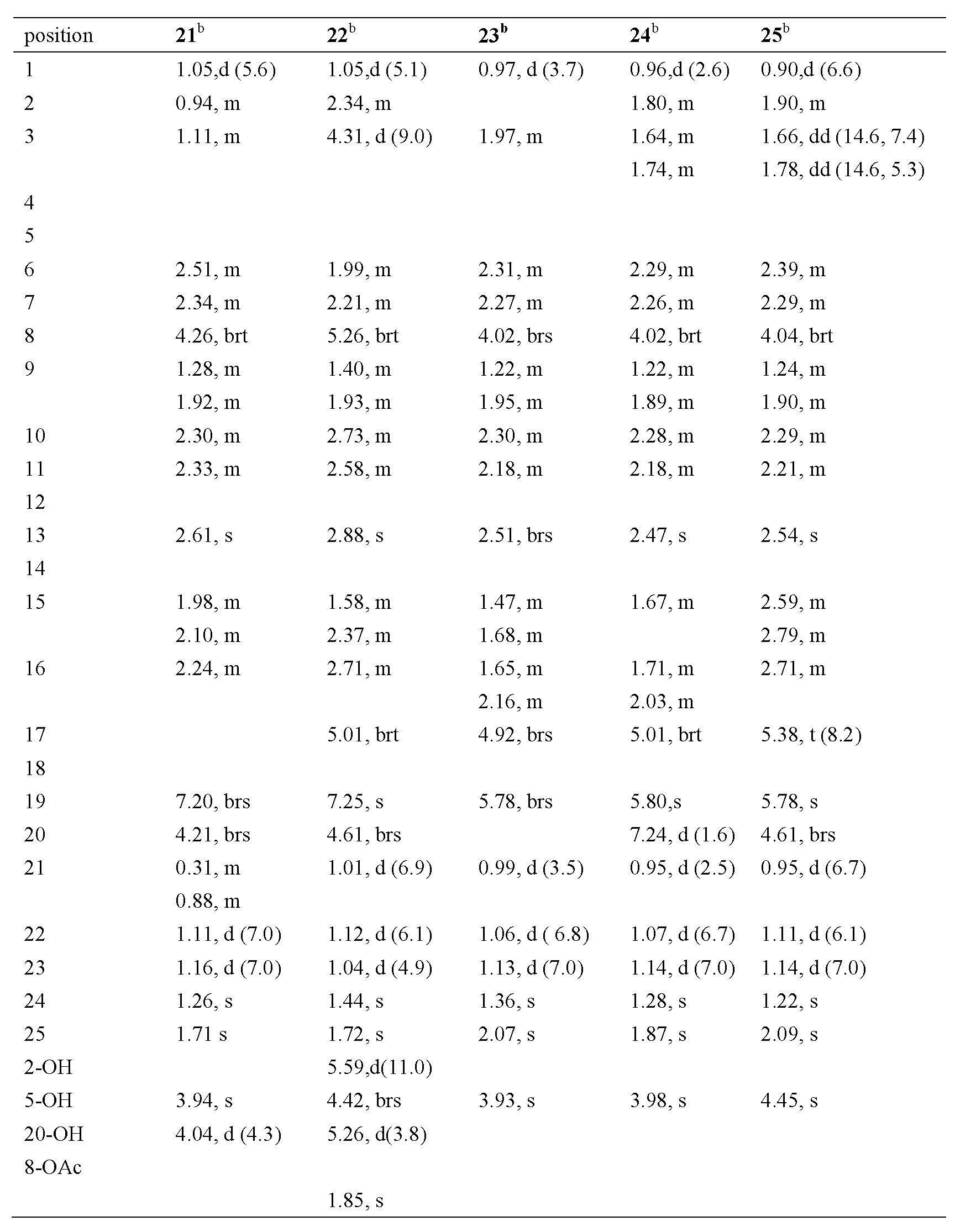Sesterterpene compound and preparation method and application thereof
A technology of disesquiterpenes and compounds, which is applied in the field of natural medicinal chemistry and achieves the effects of good anti-prolyl endopeptidase activity, fresh and pure aroma, and good angiogenesis inhibitory activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Extraction, separation and purification of compound 1-34 of the present invention:
[0040] Dry the leaves (15 kg) of the genus Torchina plant in the shade, crush them to 30 meshes, extract them with n-ethane at room temperature for 3 times (45 L each time, 24 h), combine the extracts, and concentrate the extracts under reduced pressure to obtain the leaching solution. Cream 210 g. The extract was dissolved in an appropriate amount of chloroform / acetone, and then mixed with silica gel (200-300 mesh), and then 1.5 kg of silica gel (200-300 mesh) was used for column chromatography to divide the crude fraction, and petroleum ether / chloroform (1:0 — 0:1) and chloroform / acetone (1:0 — 0:1) for gradient elution to obtain 8 major fractions. A total of 45 g of the chloroform and chlorine / propane (9:1) fractions were subjected to medium-pressure liquid phase MCI column chromatography, and gradient elution was performed with acetone / water (70% — 100%), of which 80% and 90% were ...
Embodiment 2
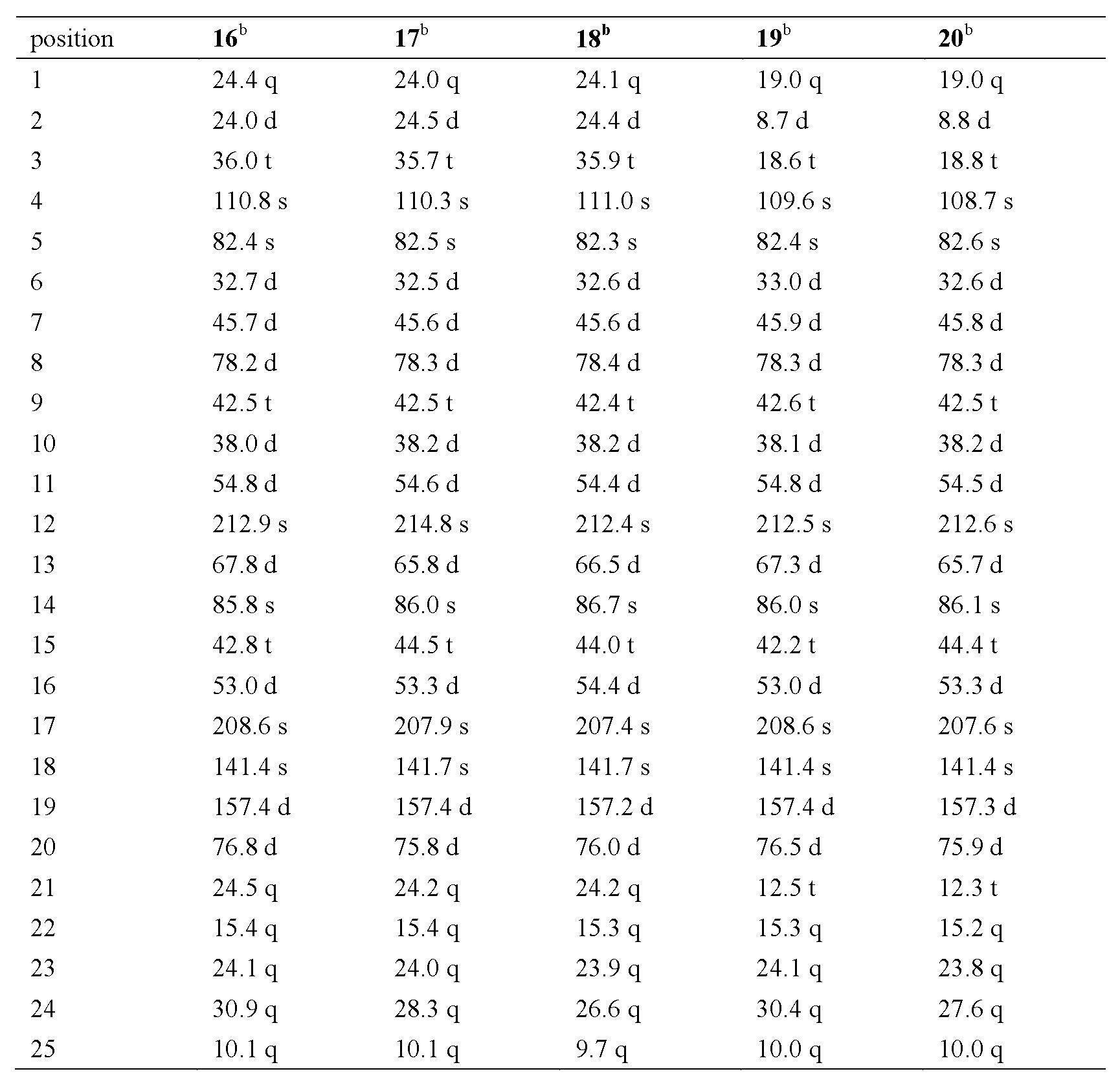
[0043] Physical and Spectroscopic Data of Compounds 1-34 of the Invention:
[0044] Compound 1: The compound is a colorless oil: [α] D 23.3 = +0.10 (c = 0.20, MeOH); UV (MeOH) λ max (log ε): 211 (2.93) nm; IR (KBr) ν max : 3440.6, 2957, 2930, 2869, 1686, 1630, 1380, 1025 cm -1 ; EI: m / z (%) 414 ([M] + , 35), 179 (30), 95 (54), 83 (100); HR-EI-MS: m / z 414.2397 (calcd for C 25 h 34 o 5 , 414.2406); 1 H and 13 See Table-1 and Table-2 for C NMR data.
[0045] Compound 2: This compound is colorless and transparent massive crystals: [α] D 26.3 = +1.07 (c = 0.15, MeOH); UV (MeOH) λ max (log ε): 217 (2.66) nm; IR (KBr) ν max : 3442, 2956, 2927, 2868, 1687, 1629, 1454, 1027, 1025, 725 cm -1 ; EI: m / z (%) 414 ([M] + , 25), 178 (25), 95 (100), 83 (75), 81 (51); HR-EI-MS: m / z 414.2398 (calcd for C 25 h 34 o 5 , 414.2406); 1 H and 13 See Table-1 and Table-2 for C NMR data.
[0046] Compound 3: This compound is white powder: [α] D 14.1 = +1.20 (c = 0.15, MeOH); UV (...
Embodiment -3
[0129] Antifeedant activity detection of the compound of the present invention:
[0130] The antifeedant activity of the compound of the present invention against beet armyworm (Spodoptera exigua) and cotton bollworm (Helicoverpa armigera) was determined by using the selective antifeedant activity assay method. Before the activity test, the larvae whose growth was relatively consistent at about the third instar were starved for 4-5 hours. The leaves of Chinese cabbage (Brassica chinensis) with consistent growth were selected, rinsed, and punched with a hole punch (1.1 cm in diameter) to make round leaves (leaf butterflies). The compound of the present invention is configured into 5 different concentration gradients with acetone, and 10 μl is dropped on the leaf butterfly, and dried to treat the leaf butterfly; for the control leaf butterfly, 10 μl of acetone solution is directly dropped on the leaf butterfly, and dried , and then put 2 treatment leaf butterflies and 2 control...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com