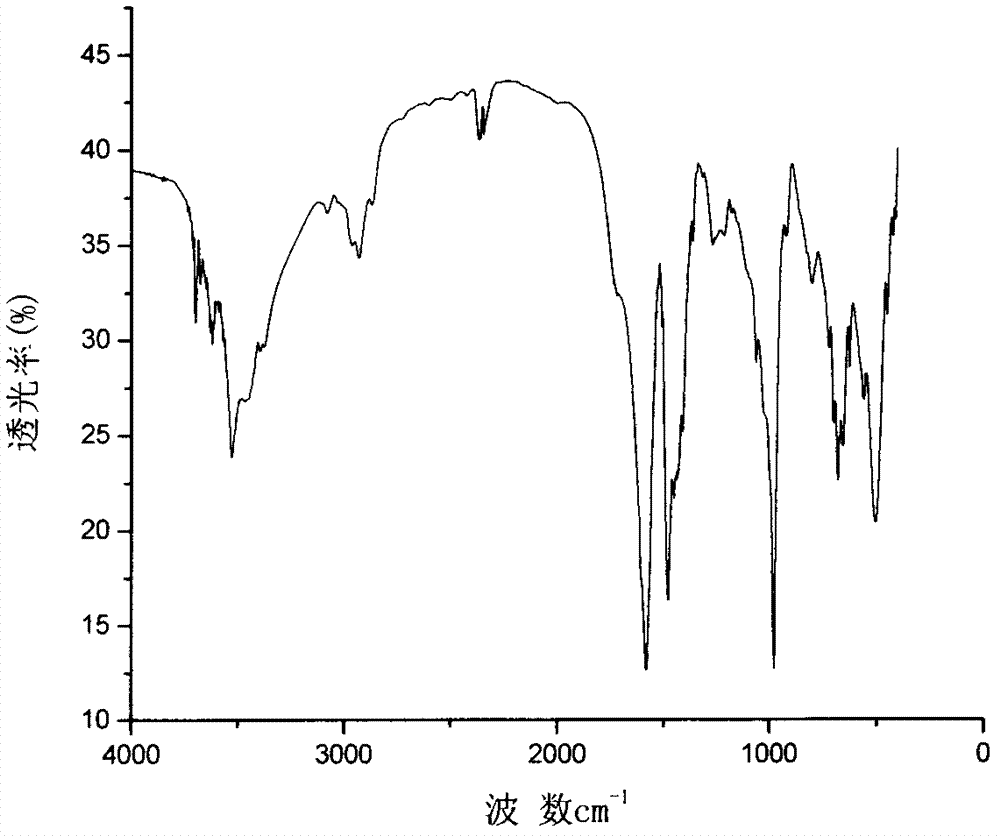
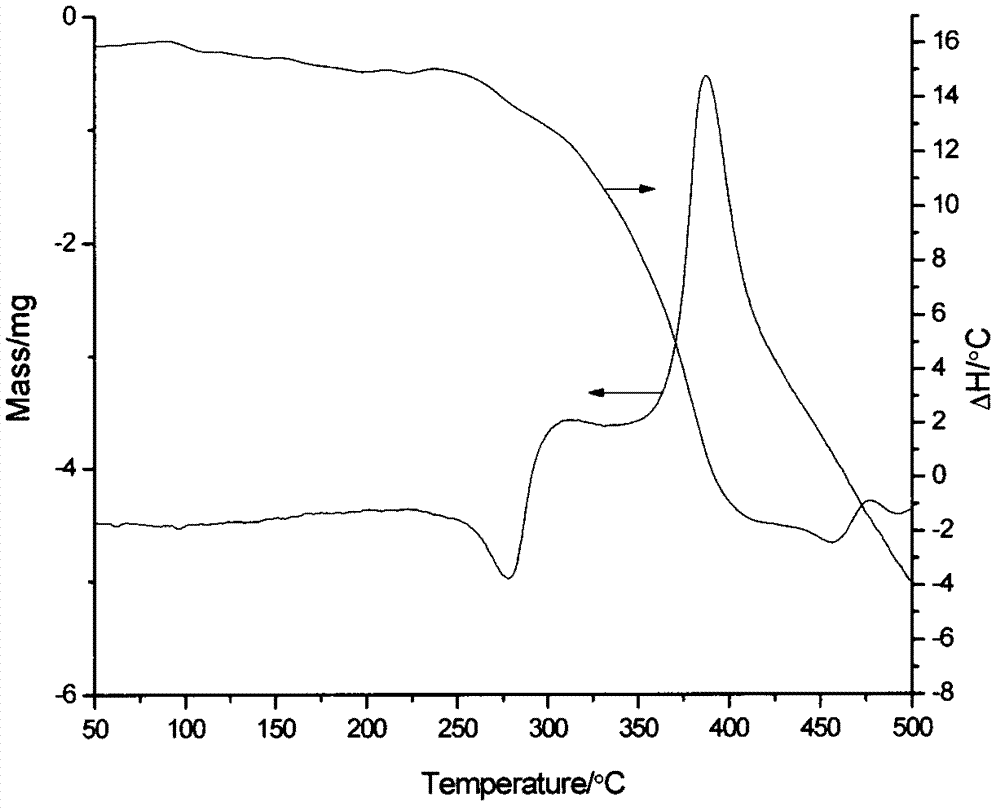
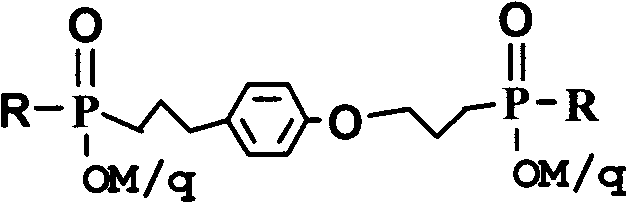
3-alkyl phosphinic propyl phenyl-3-alkyl phosphinic propyl ether metal salts and preparation method thereof
A technology of alkylphosphinyl propyl phenyl and alkyl phosphinyl propyl ether, which is applied in the field of organic phosphinous compounds and their preparation, can solve problems such as hindering industrial development and lagging behind, and achieve the goal of not producing three wastes pollution, Good thermal stability, easy charring and anti-dripping effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Example 1 Self-made anhydrous hypophosphorous acid-acetic acid solution, add 25.4g (0.24mol) sodium hypophosphite monohydrate and 100ml acetic acid into a 500ml three-neck flask, stir and dissolve, add 12g concentrated sulfuric acid dropwise, stir for 0.5h, cool and filter Remove sodium sulfate hydrate, wash the filter cake with 100ml acetic acid, and take the filtrate to obtain anhydrous hypophosphorous acid-acetic acid solution.
[0032] The resulting anhydrous hypophosphorous acid-acetic acid solution was added to a 500ml three-necked flask, heated to 90°C, and then 20.9g (0.12mol) of allylphenyl allyl ether and 1.16g of benzoyl peroxide were dissolved. Dissolve it in 100ml of acetic acid, and slowly add it to a three-necked flask within 6 hours. After the addition, keep it warm for 2 hours, cool it down, pour all the reaction solution into a 500ml high-pressure reactor, exhaust the air in the reactor, and heat up to 90°C, start to feed ethylene, set the pressure of ...
Embodiment 2
[0033] Example 2 Self-made anhydrous hypophosphorous acid-acetic acid solution, the operation method is the same as that in Example 1. Add 0.24mol of self-made anhydrous hypophosphorous acid-acetic acid solution into a 500ml three-necked flask, heat up to 110°C, and then dissolve 20.9g (0.12mol) allyl phenyl allyl ether and 0.94g tert-butyl peroxybenzoate are dissolved in 100ml acetic acid, and it is slowly added in the there-necked flask in 6h, after adding, keep warm for 2h, cool, and All the reaction solution is poured into a 500ml high-pressure reactor, the air in the reactor is exhausted, the temperature is raised to 110° C., and ethylene is started to be introduced. The acetic acid solution of tert-butyl oxide is poured into the reaction kettle with a constant flow pump, and the temperature is controlled not to exceed 115°C. When the ethylene consumption no longer increases, the constant flow pump is turned off, and the reaction is carried out at constant temperature and ...
Embodiment 3
[0034] Example 3 Self-made anhydrous hypophosphorous acid-acetic acid solution, the operation method is the same as that in Example 1. Add 0.18mol of self-made anhydrous hypophosphorous acid-acetic acid solution into a 500ml three-necked flask, heat up to 90°C, and then dissolve 20.9g (0.12mol) allyl phenyl allyl ether and 0.6 g of azobisisobutyronitrile were dissolved in 100 ml of acetic acid, and slowly added to the three-necked flask within 6 hours. Pour it into a 500ml high-pressure reactor, exhaust the air in the reactor, heat up to 90°C, start feeding ethylene, set the pressure of the ethylene pressure reducing valve to 0.6MPa, and simultaneously inject 0.6g of azobisisobutyronitrile The acetic acid solution was poured into the reaction kettle with a constant flow pump, and the temperature was controlled not to exceed 95°C. When the ethylene consumption no longer increased, the constant flow pump was turned off, and the reaction was carried out at constant temperature and...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com