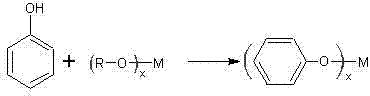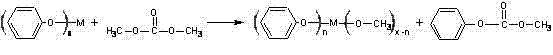Method for synthesizing diphenyl carbonate from dimethyl carbonate
A technology of dimethyl carbonate and diphenyl carbonate, which is applied in the field of synthesizing diphenyl carbonate, can solve the problems of difficult reaction, harsh process conditions, complex and cumbersome process, etc., and achieve the effects of easy control, high reaction efficiency and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
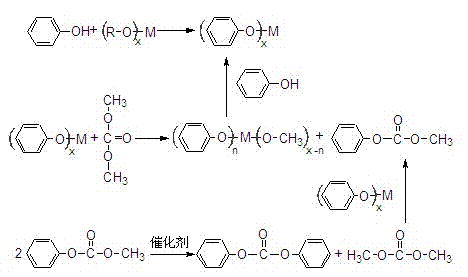
[0034] Step 1: After dehydration, the phenol and butyl titanate are mixed evenly in a molar ratio of 4:1, and phenyl titanate is synthesized by reaction distillation under normal pressure. The reaction temperature is 150°C, and the reaction time is 2 hours. Alcohol, isolated from phenyl titanate.
[0035] Step 2: Phenyl titanate and dimethyl carbonate are mixed in a molar ratio of 1:4, and the reaction is carried out in a closed reactor with a pressure of 5 atm, a reaction temperature of 150°C, and a reaction time of 4 hours to obtain methyl Phenyl carbonate.
[0036] Step 3: The obtained product methyl phenyl carbonate carries out disproportionation reaction, adopts atmospheric pressure reaction distillation process, and reaction temperature is 180 ℃, and catalyst is phenyl titanate, and its content is 4wt%, and reaction time is 4 hours, obtains dicarbonate phenyl esters.
[0037] Step 4: Separating the reaction mixture in step 2 under reduced pressure to finally obtain met...
Embodiment 2
[0039] Step 1: After dehydration, phenol and isopropyl titanate are mixed evenly in a molar ratio of 4:1, and phenyl titanate is synthesized by reaction distillation under normal pressure. The reaction temperature is 140°C, and the reaction time is 3 hours. Butanol, isolated from phenyl titanate.
[0040] Step 2: Phenyl titanate and dimethyl carbonate are mixed at a molar ratio of 1:4 and reacted in a closed reactor with a pressure of 8atm, a reaction temperature of 160°C, and a reaction time of 4 hours to obtain formazan phenyl carbonate.
[0041] Step 3: The obtained product methyl phenyl carbonate is subjected to disproportionation reaction, adopts atmospheric pressure reactive distillation process, the reaction temperature is 180 ° C, the catalyst is phenyl titanate, its content is 4wt%, and the reaction time is 4 hours to prepare carbonic acid diphenyl esters.
[0042] Step 4: Separating the reaction mixture in step 2 under reduced pressure to finally obtain methyl tita...
Embodiment 3
[0044] Step 1: After dehydration, phenol and isopropyl titanate are mixed evenly in a molar ratio of 4:1, and phenyl titanate is synthesized by reaction distillation under normal pressure. The reaction temperature is 130°C, and the reaction time is 3 hours. Butanol, isolated from phenyl titanate.
[0045] Step 2: Phenyl titanate and dimethyl carbonate are mixed at a molar ratio of 1:4 to react. The reaction is carried out in a closed reactor with a pressure of 10atm, a reaction temperature of 150°C, and a reaction time of 4 hours to obtain formazan phenyl carbonate.
[0046] Step 3: The obtained product methyl phenyl carbonate is subjected to disproportionation reaction, adopts atmospheric pressure reactive distillation process, the reaction temperature is 180 ° C, the catalyst is phenyl titanate, its content is 4wt%, and the reaction time is 4 hours to prepare carbonic acid diphenyl esters.
[0047] Step 4: Separating the reaction mixture in step 2 under reduced pressure to...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com