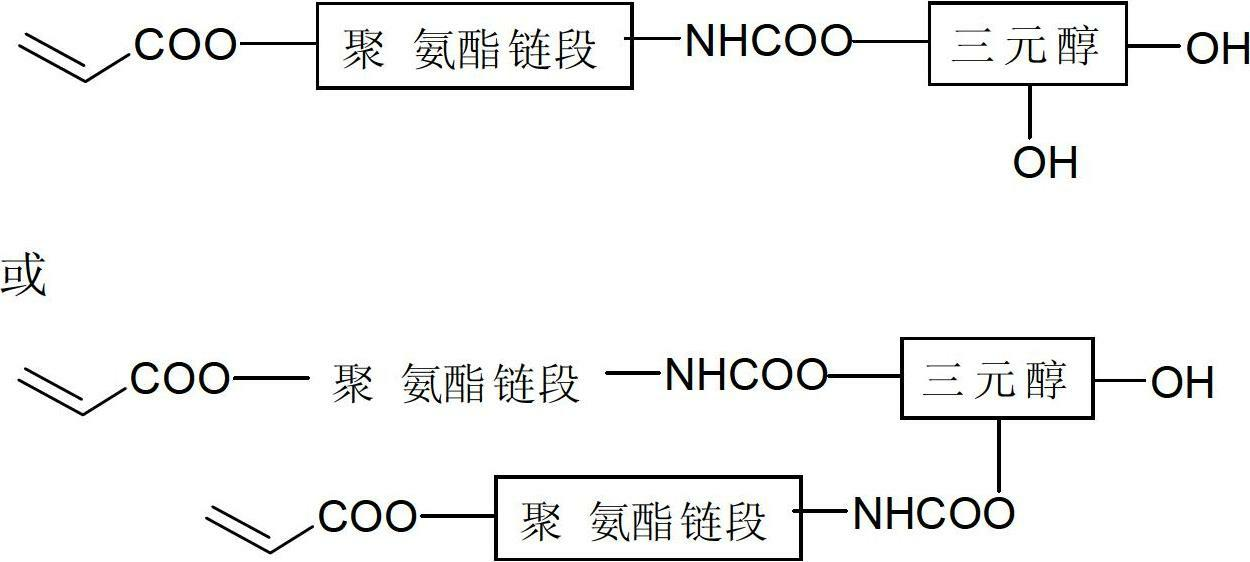
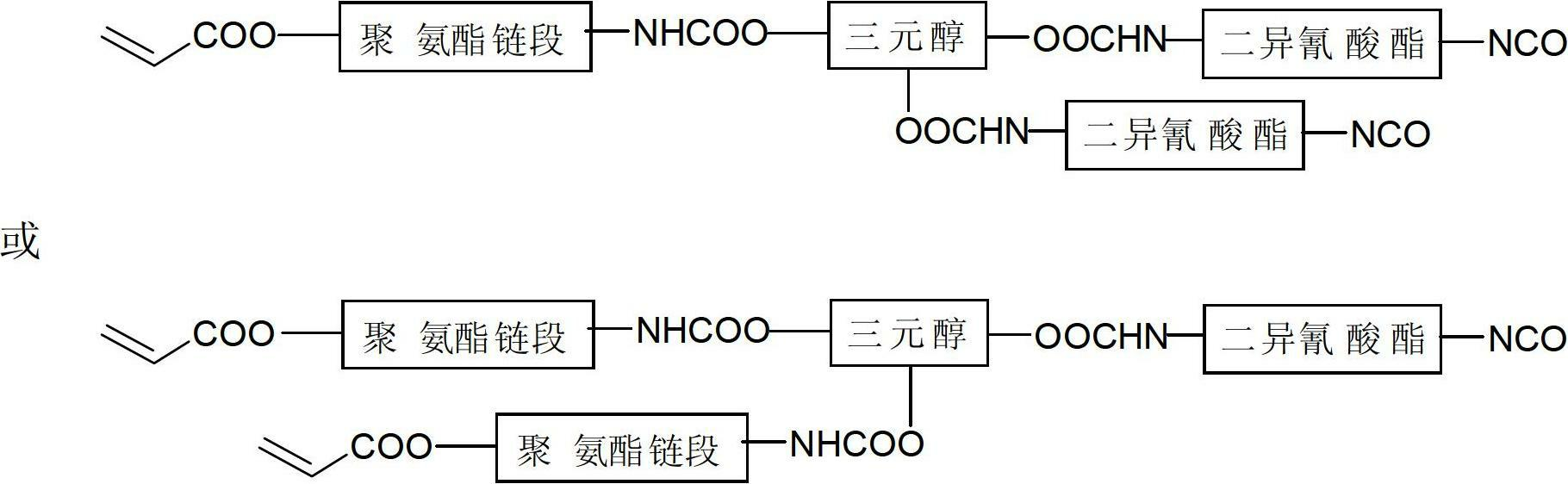
Preparation method for alicyclic epoxy and double bond contained dual-curing resin and application thereof
A dual-curing, dual-curing technology, used in applications, household appliances, adhesive types, etc., can solve the problems of unstable cured film performance, inconsistent polymerization rate, poor comprehensive performance, etc., and achieve good UV cationic curing activity, The effect of improving performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] In the first step, add 1mol of toluene diisocyanate (TDI) into a 500mL flask, stir, heat to 50°C, and add 0.3% of dibutyl dilaurate to 0.5mol of 1,4-butanediol. Tin acid, dropwise added toluene diisocyanate (TDI), reacted for 3 hours, then added 0.5 mol of hydroxyethyl acrylate (dissolved with 0.5% of the total mass of p-hydroxyanisole), and raised the temperature to 65 ° C to continue the reaction for 3 hours; In the second step, add 0.5mol of glycerol to a 500mL flask, and add 0.2% of the total mass of dibutyltin dilaurate, heat to 60 ° C, and synthesize the polyurethane containing 0.5mol of NCO groups in the first step The acrylate segment is slowly added dropwise into glycerol, and reacted for 2.5 hours to obtain the prepolymer ①; the third step: add 1mol toluene diisocyanate (TDI) to a 1000mL flask, and add 0.2% of the total mass of dibutyl Tin dilaurate, heated to 70°C, slowly add the prepolymer ① containing 1molOH synthesized in the second step into the diisocyan...
Embodiment 2
[0027]In the first step, add 2mol of diphenylmethane diisocyanate (MDI) in a 1000mL flask, stir, heat to 60°C, add 0.4% of the total mass of dibutyl dilauric acid to 1mol of polyether diol Tin, add methane diisocyanate (MDI) dropwise, after reacting for 4 hours, then add 1 mol of hydroxyethyl methacrylate (dissolved with 0.6% p-hydroxyanisole in total mass), heat up to 70°C and continue the reaction for 2.5 hours; In the second step, add 1mol of trimethylolpropane to a 500mL flask, and add 0.3% of the total mass of dibutyltin dilaurate, heat to 50 ° C, and synthesize the first step containing 2mol of NCO groups The urethane acrylate segment is slowly added dropwise into trimethylolpropane, and reacted for 4 hours to obtain a prepolymer ①; the third step: add 1mol diphenylmethane diisocyanate (MDI) to a 500mL flask, and add 0.1 % dibutyltin dilaurate, heated to 65°C, slowly added the prepolymer ① containing 1molOH synthesized in the second step into the diisocyanate, and reacte...
Embodiment 3
[0029] In the first step, add 1mol of hexamethylene diisocyanate (HDI) into a 500mL flask, stir, heat to 55°C, add 0.5% of the total mass of dibutyl to 0.5mol of 1,6-hexanediol Tin dilaurate, add hexamethylene diisocyanate (HDI) dropwise, react for 3 hours, then add 0.5mol of hydroxypropyl acrylate (dissolved with 0.8% of the total mass of p-hydroxyanisole), and heat up to 65°C Continue to react for 3h; in the second step, add 0.5mol of trimethylolpropane to a 500mL flask, and add 0.1% of the total mass of dibutyltin dilaurate, heat to 65 ° C, and synthesize in the first step The urethane acrylate segment containing 0.5mol NCO group is slowly added dropwise into trimethylolpropane, and reacted for 4 hours to obtain the prepolymer ①; the third step: add 1mol hexamethylene diisocyanate (HDI) to a 1000mL flask, And add 0.3% of the total mass of dibutyl tin dilaurate, heat to 60 ° C, slowly add the prepolymer ① containing 1molOH synthesized in the second step to the diisocyanate, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com