Method for Determination of Soluble Mercury and Valence Mercury in Drugs Containing Cinnabar
A soluble and cinnabar technology, applied in the direction of measuring devices, preparation of test samples, and material analysis by electromagnetic means, can solve the problems of lack of scientific basis for usage and dosage, unknown material basis, and difficulty in rationally evaluating safe doses, etc., to achieve filling Domestic blank, sensitive effect of the method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
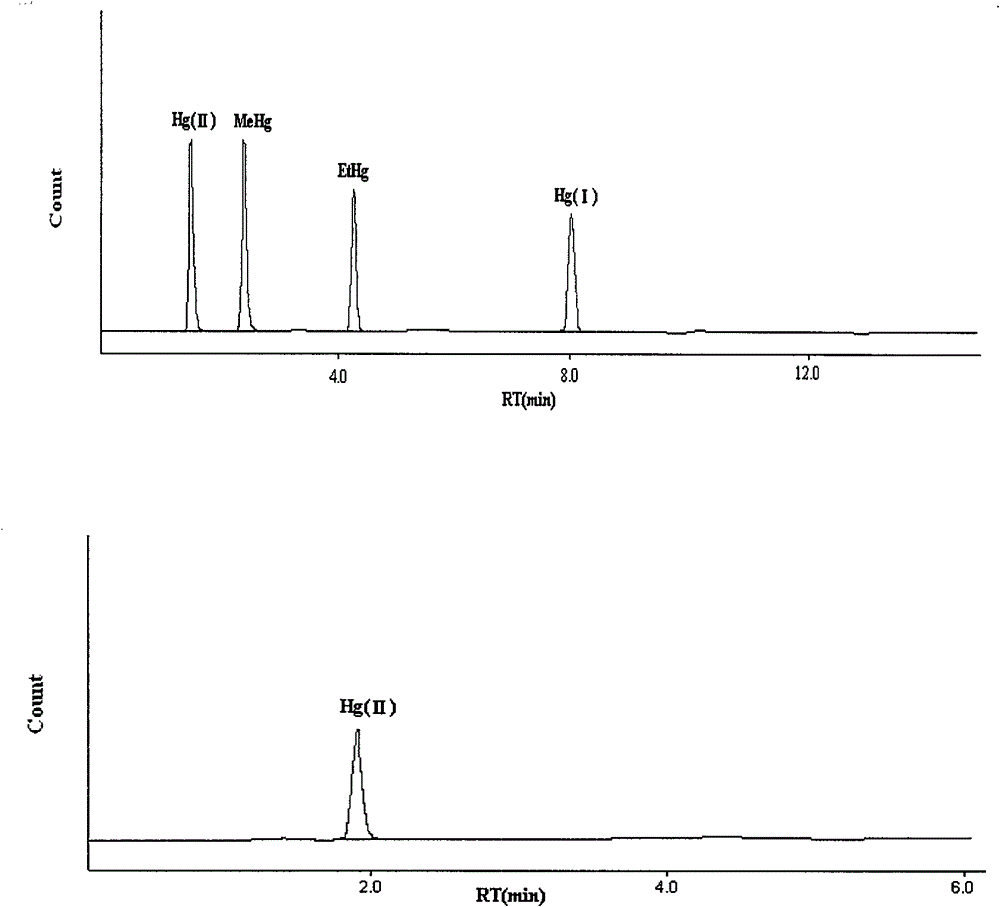
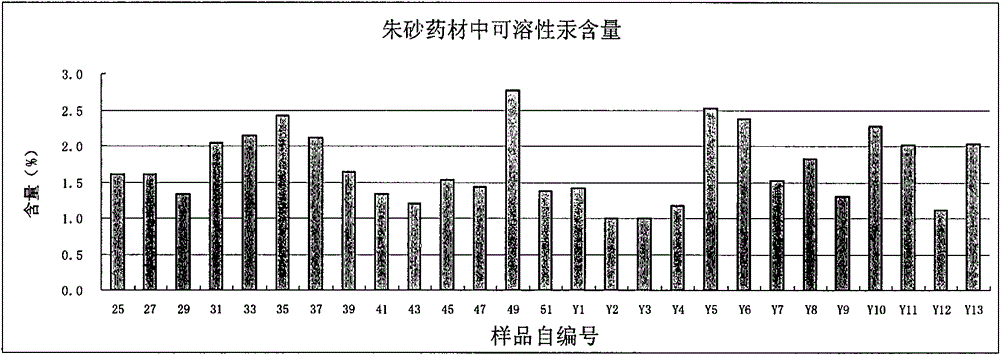
[0062] Determination of soluble mercury content in cinnabar medicinal materials: according to inductively coupled plasma mass spectrometry (Appendix XI D of Chinese Pharmacopoeia 2010 edition).
[0063] Reagents and Instruments
[0064] 7700ce inductively coupled plasma mass spectrometer from Agilent, USA; B9500S-DTH ultrasonic cleaner (power 300W, frequency 45kHz) from Branson Ultrasound (Shanghai) Co., Ltd.; Milli-Q ultrapure water machine from Millipore, USA. Mercury standard solution (1000 mg / L, batch number HC931554), purchased from Merk Company.
[0065] Nitric acid and hydrochloric acid were both excellent grade pure (Merck company); pepsin was a biochemical reagent, the enzyme activity was 1:27000, and the batch number was 100710, purchased from Shanghai Huixing Biochemical Reagent Co., Ltd.; trypsin was a biochemical reagent, casein converting capacity ≥25.0, the batch number is F20100714, purchased from Sinopharm Chemical Reagent Co., Ltd.; the water is deionized wa...
Embodiment 2
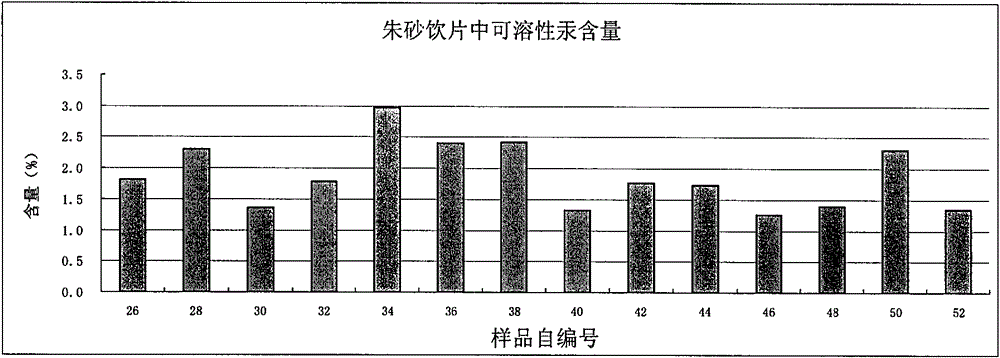
[0110] Determination of soluble mercury content in cinnabar decoction pieces: according to inductively coupled plasma mass spectrometry (Appendix XI D of Chinese Pharmacopoeia 2010 edition).
[0111] The cinnabar medicinal material in Example 1 was replaced with cinnabar decoction pieces (derived from Guizhou Tongren Mining Industry, processed by Shanghai Fengbang Traditional Chinese Medicine Piece Factory, batch number H2008030502), and other methods and processes were the same as in Example 1.
[0112] Solution stability test:
[0113] Get the cinnabar decoction pieces, prepare the test solution according to the same method of preparing the test solution in Example 1 "Determination of the content of soluble mercury in the medicinal material of cinnabar", and analyze the samples at different times to measure the amount of soluble mercury, the results show (see table 4), the soluble mercury content in the test solution is basically stable within 0 to 24 hours.
[0114] Table ...
Embodiment 3
[0151] Determination of soluble mercury content in cinnabar preparations: according to inductively coupled plasma mass spectrometry (Appendix XI D of Chinese Pharmacopoeia 2010 edition).
[0152] Preparation of the test solution: take Jufang Zhibao Powder (provided by Wuhan Jianmin Dapeng Pharmaceutical Co., Ltd., batch No. 4) 150mg, grind finely, accurately weigh, (1) put a 250ml glass measuring bottle (first use 20% hydrochloric acid) The solution (v / v) was soaked overnight, then washed with deionized water and air-dried), added artificial intestinal juice to the volume, shaken well, tightly plugged, weighed, and placed at 37°C for ultrasonic treatment (power 300W, Frequency 45kHz) for 2 hours, shake once every 10-15 minutes, or (2) put a 250ml ~ 500ml conical flask with stopper (soak in 20% hydrochloric acid solution (v / v) overnight, then use deionized water Wash and dry), add 250ml of artificial intestinal juice, shake well, weigh the fixed weight, place it in a 37°C water...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com