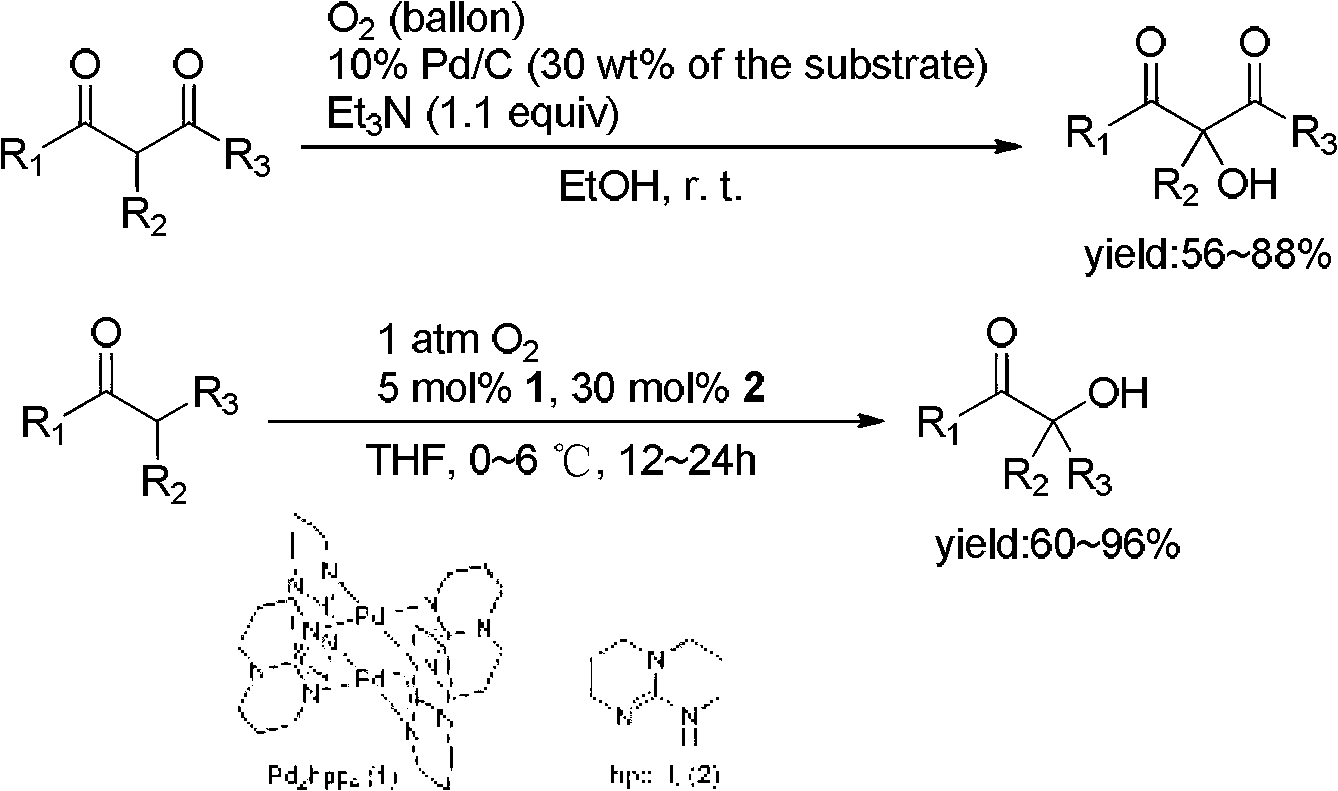
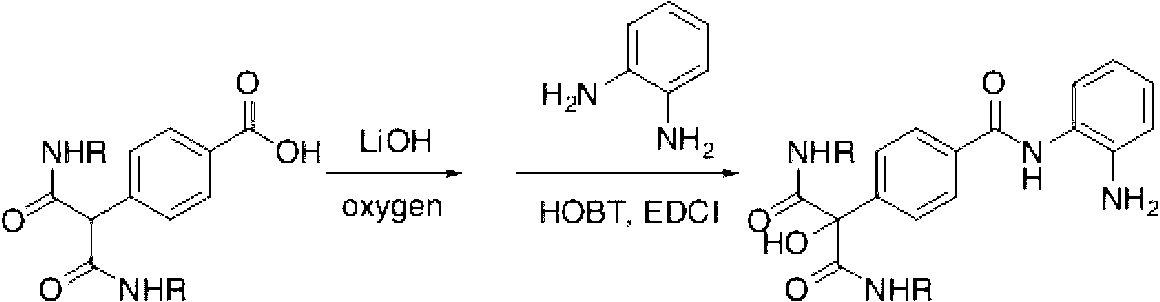
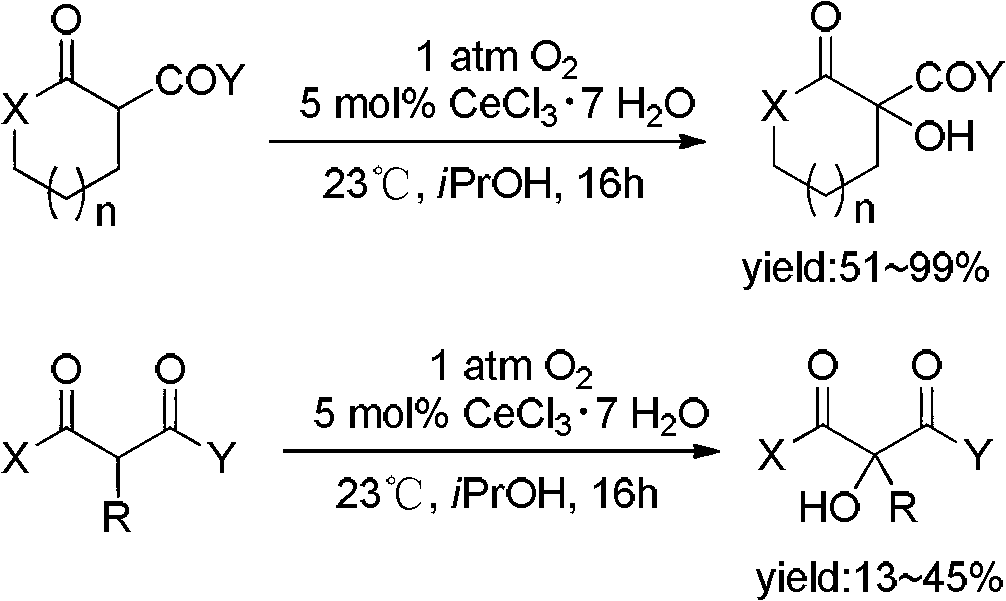Method for direct alpha-hydroxylation by beta-dicarbonyl compound under action of iodine catalysis
A dicarbonyl compound and hydroxylation technology is applied in the field of direct α-position hydroxylation of β-dicarbonyl compounds catalyzed by iodine, which can solve the problems of harsh reaction conditions, complicated operations, expensive catalysts, etc., and achieves simple product separation and extended application range. , to achieve the effect of simplicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Weigh 7 (1mmol), I in "Reaction Formula 3" 2 (25.6mg, 0.01mmol) and NaOAc (4.1mg, 0.01mmol) in a 25mL round-bottomed flask, then add 10mL THF at 25°C and place it in the air and stir. The reaction was tracked by TLC. After the raw materials disappeared, most of the solvent was removed from the reactant on a rotary evaporator, and 40 mL of water and saturated Na were added. 2 S 2 O 3 Solution until the color of iodine disappears. Extracted with dichloromethane (15mL×3), the organic layer was subjected to anhydrous MgSO 4 After drying, the mixture obtained by rotary evaporation is separated by silica gel column chromatography (the eluent is petroleum ether / ethyl acetate: v / v=6 / 1). The main component obtained is product 8, and the yield is 90%.
Embodiment 2
[0040] Weigh 7 (1mmol), I in "Reaction Formula 3" 2 (128mg, 0.5mmol) and NaOAc (41mg, 0.5mmol) in a 25mL round-bottomed flask, and then add 10mL THF at 25°C and place it in the air and stir. The reaction was tracked by TLC. After the raw materials disappeared, most of the solvent was removed from the reactant on a rotary evaporator, and 40 mL of water and saturated Na were added. 2 S 2 O 3 Solution until the color of iodine disappears. Extracted with dichloromethane (15mL×3), the organic layer was subjected to anhydrous MgSO 4 After drying, the mixture obtained by rotary evaporation is separated by silica gel column chromatography (the eluent is petroleum ether / ethyl acetate: v / v=6 / 1). The main component obtained is product 8, and the yield is 95%.
Embodiment 3
[0042] Weigh 7 (1mmol), I in "Reaction Formula 3" 2 (25.6mg, 0.1mmol) and NaOAc (17mg, 0.1mmol) in a 25mL round-bottomed flask, and then add 10mL THF at 25°C and place it in the air and stir. The reaction was tracked by TLC. After the raw materials disappeared, most of the solvent was removed from the reactants on a rotary evaporator, and 40 mL of water and saturated Na2S were added. 2 O 3 Solution until the color of iodine disappears. Extracted with dichloromethane (15mL×3), the organic layer was subjected to anhydrous MgSO 4 After drying, the mixture obtained by rotary evaporation is separated by silica gel column chromatography (the eluent is petroleum ether / ethyl acetate: v / v=6 / 1). The main component obtained is product 8, and the yield is 93%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com