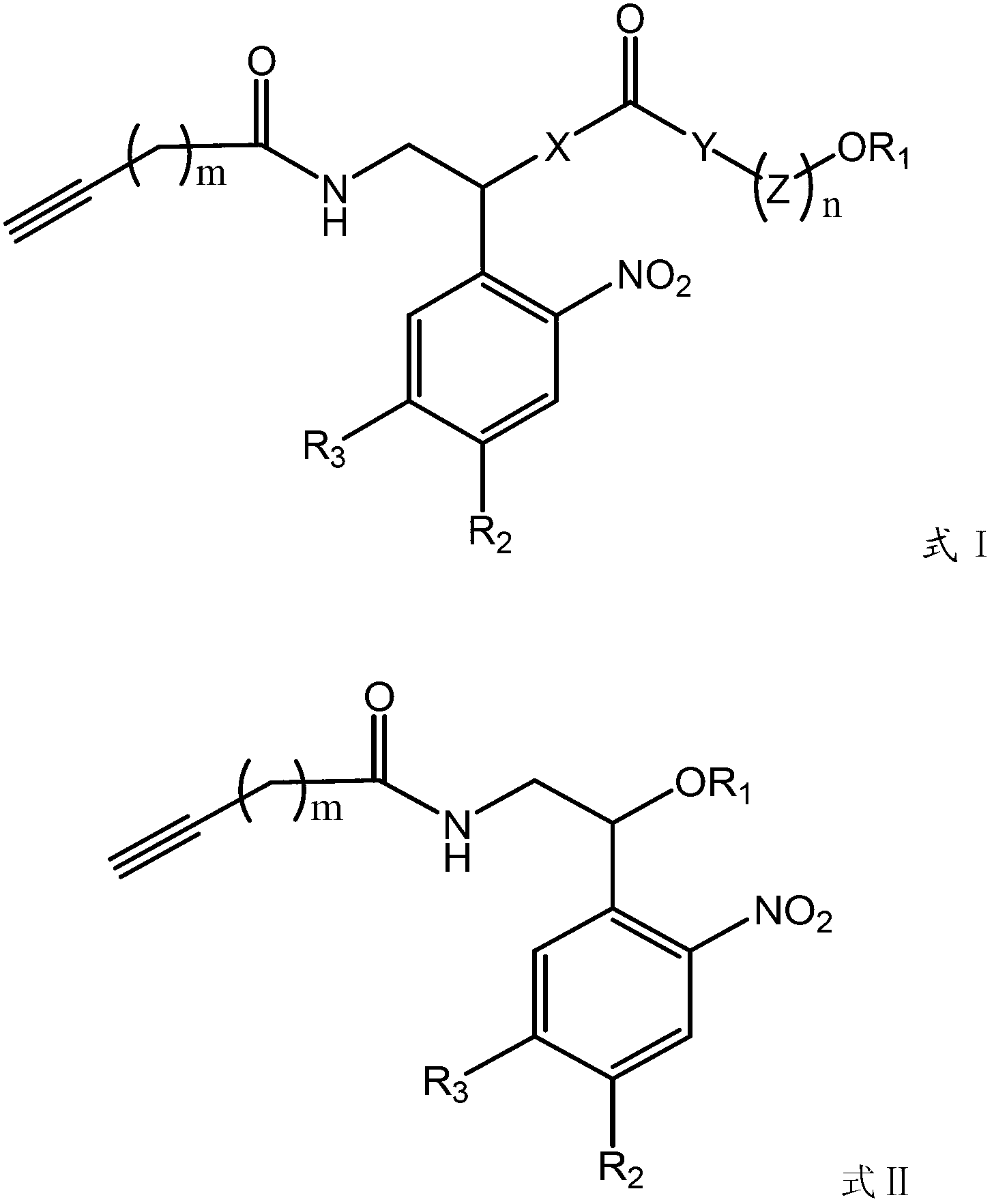
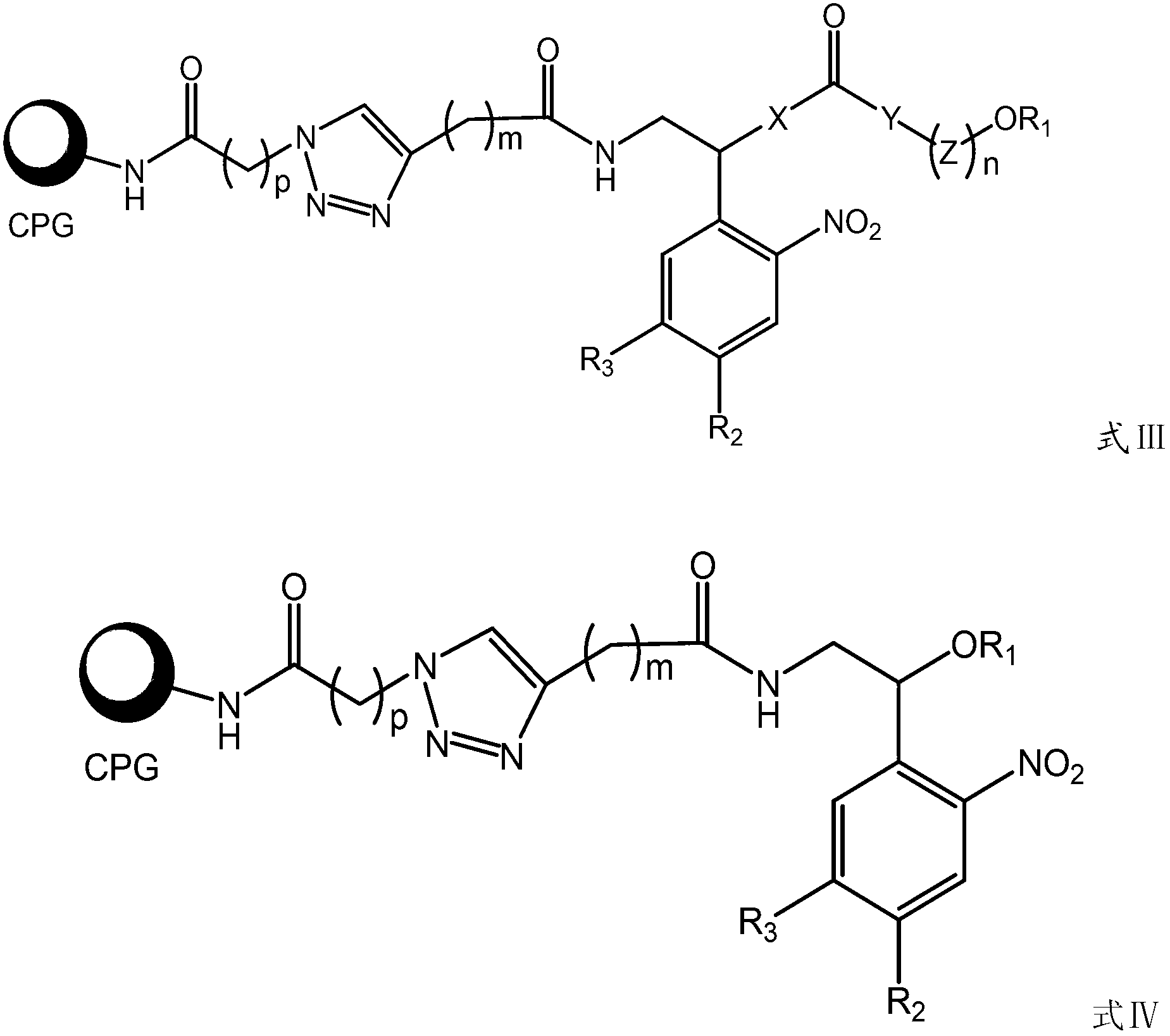
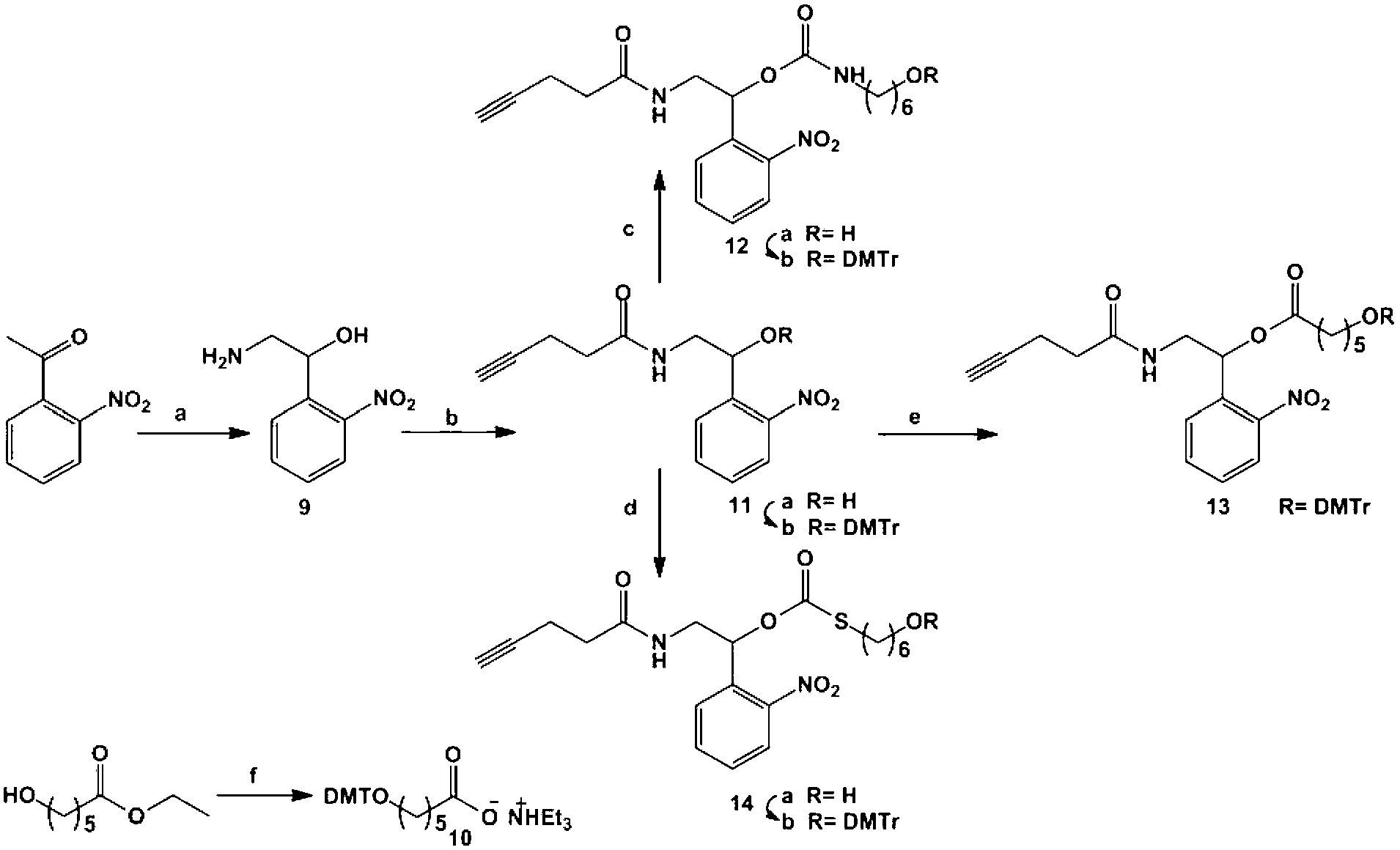
Photosensitive functionalized solid-supported phase, preparation method and application thereof
A functionalized, light-sensitive technology that can be used in biochemical equipment and methods, chemical instruments and methods, preparation of organic compounds, etc., and can solve problems such as reduced yields of oligomers
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Example 1 Preparation of 2-amino-1-(2-nitrophenyl)ethanol (compound 9)
[0051] o-Nitroacetophenone (5.00g, 30.3mmol), CuBr 2 (12.8g, 62.0mmol) was dissolved in a mixed solvent of chloroform (15mL) and ethyl acetate (10mL), heated to reflux at 80°C, and 2 drops of Br 2 Continue to react for 2 hours, add ethyl acetate (50mL) to dilute the system, wash with water (3×80mL), dry over anhydrous sodium sulfate, concentrate the organic phase, dissolve the residue in acetone (15mL), ice-bath, slowly add NaN 3 (2.36g, 36.4mmol), after the addition was completed, it was gradually raised to room temperature, and after the reaction was continued for 1 hour, ethyl acetate was added to dilute, the mother liquor was filtered and concentrated, and the residue was dissolved in methanol (10mL) and dioxane (5mL). Mixed solvent, ice bath and stirring, NaBH 4 (1.14g, 30.0mmol) was dissolved in dioxane (5mL) and added dropwise to the above system. After 10 minutes, the reaction was stopped...
Embodiment 2
[0052] Example 2 Preparation of 6-[two(4-methoxyphenyl)(phenyl)methoxy]hexanoic acid triethylamine (compound 10)
[0053] In anhydrous environment, to DMTr-Cl (two (4-methoxyphenyl) (phenyl) methyl chloride; also known as: 4,4-bismethoxytrityl chloride, 4,4- Dimethoxytrityl chloride) (500 mg, 1.48 mmol) in dichloromethane (10 mL) was added with ethyl 6-hydroxyhexanoate (200 μL, 1.23 mmol) and triethylamine (TEA) (430 μL, 3.08 mmol). The system was stirred at room temperature for 1 hour and then washed with NaHCO 3 Aqueous extraction (0.1M, 2×30mL), dried over anhydrous sodium sulfate. The organic phase was concentrated, and the residue was dissolved in aqueous NaOH (6M, 25 mL) and ethanol (4 mL), stirred at room temperature until the disappearance of the esters as monitored by TLC (thin layer chromatography). The system was diluted with chloroform (50 mL), washed with water (3×50 mL). The organic phase was concentrated and separated on a silica gel column. The ratio of mobi...
Embodiment 3
[0054] Example 3 Preparation of N-[2-hydroxyl-2-(2-nitrophenyl) ethyl] 4-yne butyramide (compound 11a)
[0055]4-Ynebutanoic acid (640mg, 6.51mmol), 1-ethyl-(3-dimethylaminopropyl) carbodiimide hydrochloride (EDC-HCl) (1.24g, 6.47mmol) and 1- Hydroxybenzotriazole (HOBt) (996mg, 6.50mmol) was dissolved in dimethylformamide (DMF) (5mL), and after stirring at room temperature for 1 hour, compound 9 (988mg, 5.40mmol) in DMF ( 3mL) solution. The system was stirred at room temperature for 3 hours, diluted with ethyl acetate (50 mL), washed with water (3×50 mL), dried over anhydrous sodium sulfate. The organic phase was concentrated and separated on a silica gel column with a mobile phase petroleum ether / ethyl acetate ratio of 1 / 1 to obtain compound 11a: light yellow oily liquid (1.13g, 4.31mmol, 80% yield). 1 H NMR (400MHz, CDCl 3 )δ=7.95(m,2H),7.67(m,1H,J=8Hz),7.45(m,1H,J=1,8Hz),6.22(s,1H),5.38(t,1H,J=4Hz ),3.70(dd,2H,J=5,6Hz).2.55(m,2H,J=2Hz),2.46(m,2H,J=2Hz),2.02(m,1H,J=3Hz)....
PUM
| Property | Measurement | Unit |
|---|---|---|
| Absorption coefficient | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com