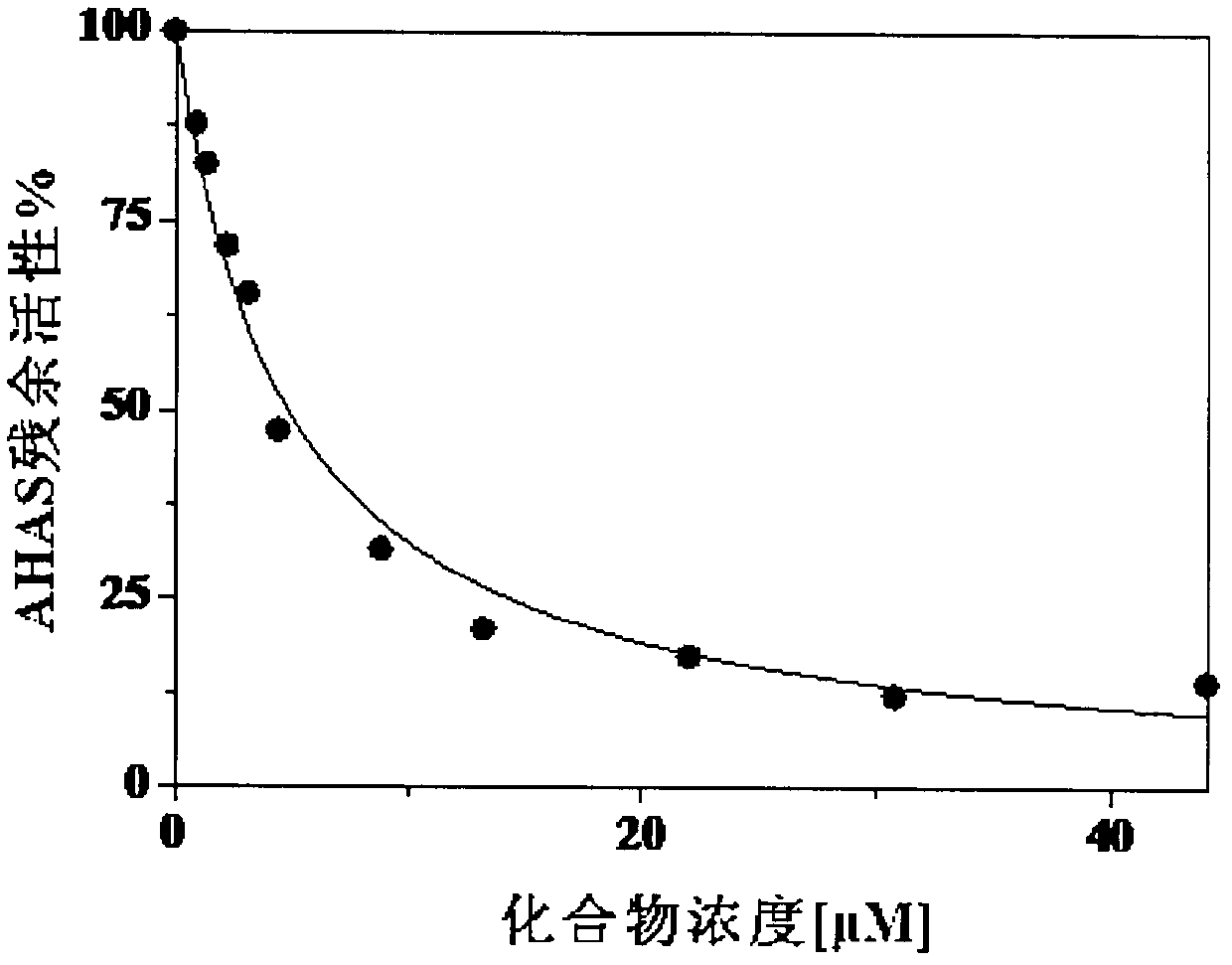
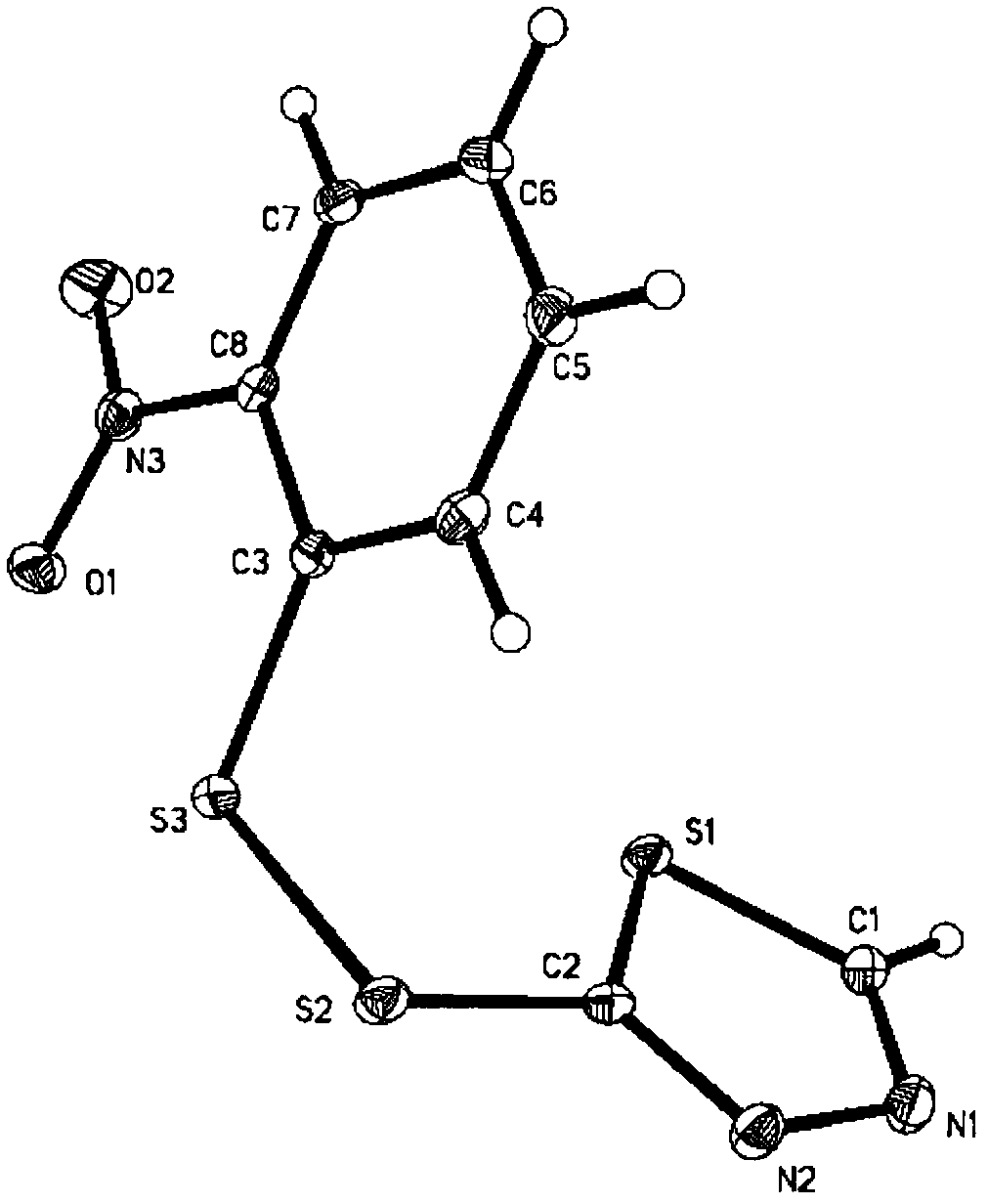
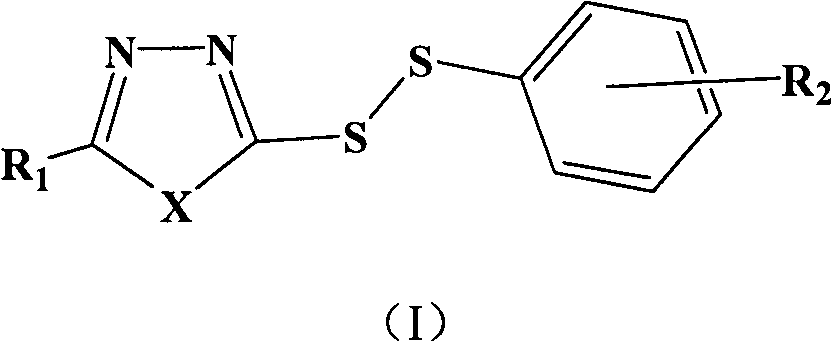
Heterocyclic asymmetric aromatic dithioether compound and synthesis method and application thereof
An asymmetric and disulfide technology, applied in the field of asymmetric aromatic disulfide compounds and herbicide preparation, to achieve good herbicidal activity and obvious inhibitory effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Embodiment 1: the preparation of benzenesulfenyl chloride
[0031]
[0032] Dissolve 1.1g (10mmol) of thiophenol in 20mL of dichloromethane, add 1.35g (10mmol) of sulfonyl chloride dropwise at the temperature of an ice-water bath, and stir for 1h. The solvent was removed in vacuo to obtain 1.36 g of red benzenesulfenyl chloride with a yield of 95%, which was directly used in the next reaction.
[0033] Similarly, p-toluenesulfenyl chloride, p-methoxybenzenesulfenyl chloride, p-bromobenzenesulfenyl chloride, p-chlorobenzenesulfenyl chloride, o-fluorobenzenesulfenyl chloride, o-toluenesulfenyl chloride, β-naphthenesulfenyl chloride.
Embodiment 2
[0034] Embodiment 2: the preparation of o-methoxycarbonylbenzenesulfenyl chloride
[0035]
[0036] Dissolve 5g (16.3mmol) of 2,2'-dithiodibenzoic acid in 37.5mL of anhydrous methanol, stir, slowly add 1.5mL of concentrated sulfuric acid dropwise, and heat to reflux for 72h. Cool to room temperature, remove excess methanol in vacuo, neutralize the concentrated solution with saturated sodium carbonate solution, bring the pH value to 7-8, extract the organic layer with ethyl acetate, wash with saturated brine, dry over magnesium sulfate, and concentrate in vacuo to obtain gray 2 , 4.5 g of solid methyl 2'-dithiodibenzoate (yield 82.5%).
[0037] The method of reference example 1 can prepare o-methoxycarbonylbenzenesulfenyl chloride.
[0038] Likewise, o-ethoxycarbonylbenzenesulfenyl chloride can be prepared.
Embodiment 3
[0039] Embodiment 3: the preparation of o-nitrobenzene sulfenyl chloride
[0040]
[0041] 1.93g (84mmol) of sodium metal was carefully and slowly added to 50mL of absolute ethanol, and after the reaction was completed, 10.33g (83mmol) of benzyl mercaptan dissolved in 25mL of absolute methanol was added. Then 13.13g (83mmol) o-nitrochlorobenzene was added dropwise, heated to reflux for 1h, cooled to room temperature, and suction filtered to obtain a yellow solid, which was then recrystallized with methanol to obtain 18.39g of 2-nitrophenylbenzyl sulfide (yield 90.3 %).
[0042] Dissolve 6.8g (50mmol) of sulfonyl chloride in 25mL of chloroform, and slowly add it dropwise to 12.2g (50mmol) of 2-nitrophenylbenzyl sulfide in 80mL of chloroform under stirring at room temperature. After the dropwise addition, the temperature was raised to 45°C. At this time, the reactants were completely dissolved, and the reaction was controlled at this temperature for 0.5 h. The solvent was re...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com