Synthesis method of vinpocetine
A technology of vinpocetine and its synthetic method, which is applied in the field of vinpocetine synthesis, can solve the problems of non-environmental protection, toxicity, and high yield, and achieve the effects of less environmental pollution, short reaction steps, and low energy consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
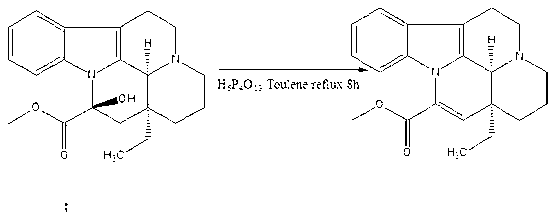
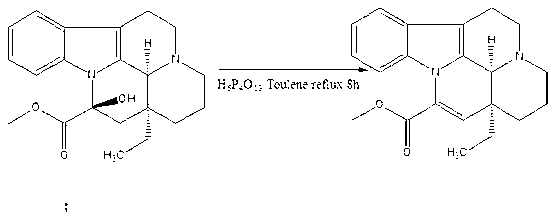
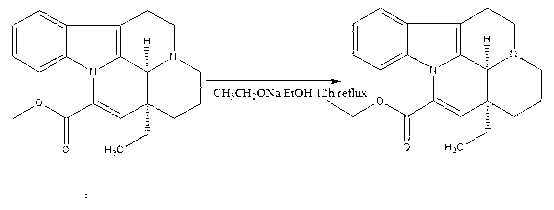
[0015] Embodiment: Vinpocetine is synthesized according to the following steps
[0016] The first step: Add the raw material vincamine (500g, 1.41mol) into a 5L three-necked flask, then add toluene (2L), stir in an ice-water bath, slowly add dropwise polyphosphoric acid (134g, 0.4mol), 0.5 After 1 hour, place it in an oil bath, install a water separator and a condenser, vacuumize, replace nitrogen twice, set the temperature at 120°C, which is slightly higher than the boiling point of toluene, and react for 8 hours. The water in the water container is released; after the reaction, evaporate the solvent to dryness, add ethanol and water (2000ml: 200ml), and add 2mol / L sodium hydroxide solution dropwise at the same time, stop the dropwise addition when PH=9, and use saturated potassium carbonate The solution was adjusted to PH=12, solids were precipitated, filtered, and dried in vacuo to obtain apovencumin (446 g, 1.33 mol) with a yield of 94%;
[0017] Step 2: Add absolute etha...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com