Preparation method of sitagliptin
A technology of sitagliptin and trifluorophenyl, applied in the field of compound preparation, can solve the problems of low optical purity ee%, waste of resources and high cost, and achieve the effects of high purity, mild process conditions and high EE value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
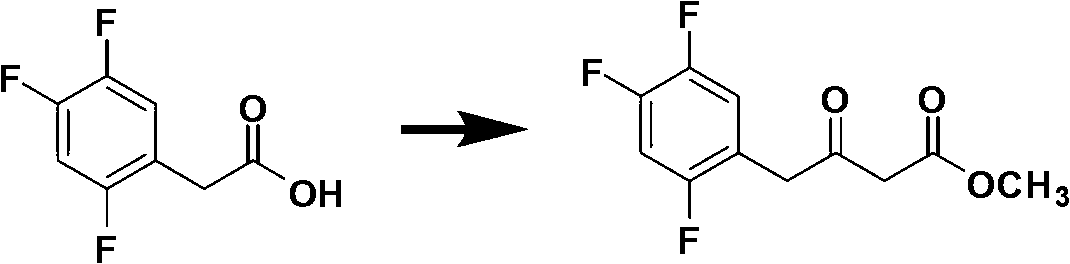
[0065] Add oxalyl chloride to the dichloromethane solution of 2,4,5-trifluorophenylacetic acid, stir for 2 hours, slowly add this solution dropwise to the dichloromethane solution of Meldrum's acid and sym-collidine, and react at zero temperature 3-6 hours. Hydrochloric acid was added to quench the reaction, and the layers were separated. Sodium hydroxide solution was added to the organic layer, the layers were separated, the organic phase was discarded, the aqueous phase was acidified with hydrochloric acid, and filtered to obtain a yellow solid. Dissolve the yellow solid in methanol, heat and reflux for 3-6 hours, and cool to obtain the methanol solution of the first step product.
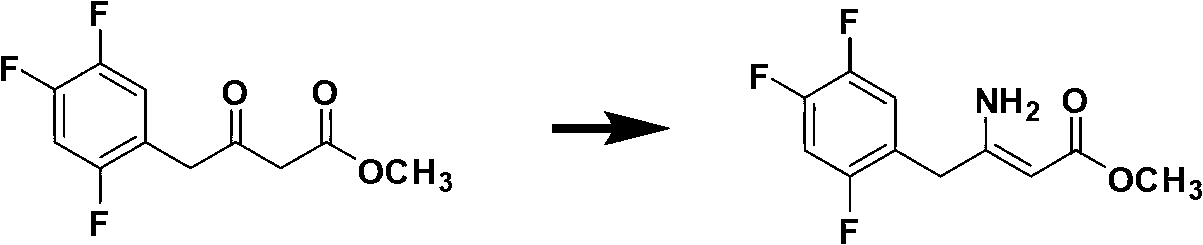
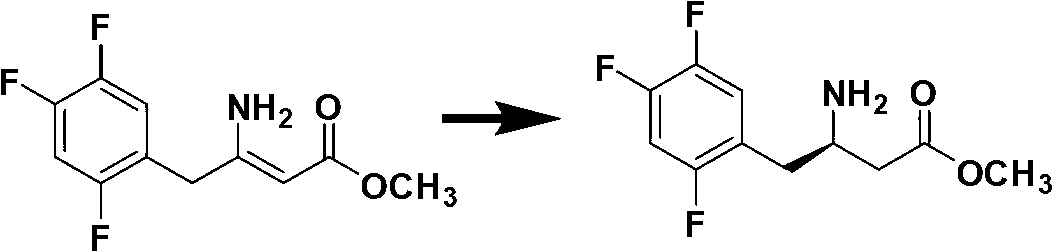
[0066] Add ammonium acetate to the methanol solution of the first step product, heat and reflux for 20 hours, cool, concentrate under pressure, add ethyl acetate after the methanol is removed, cool, separate the ammonium acetate slurry layer, wash once with water, and distill ethyl acetate under...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com