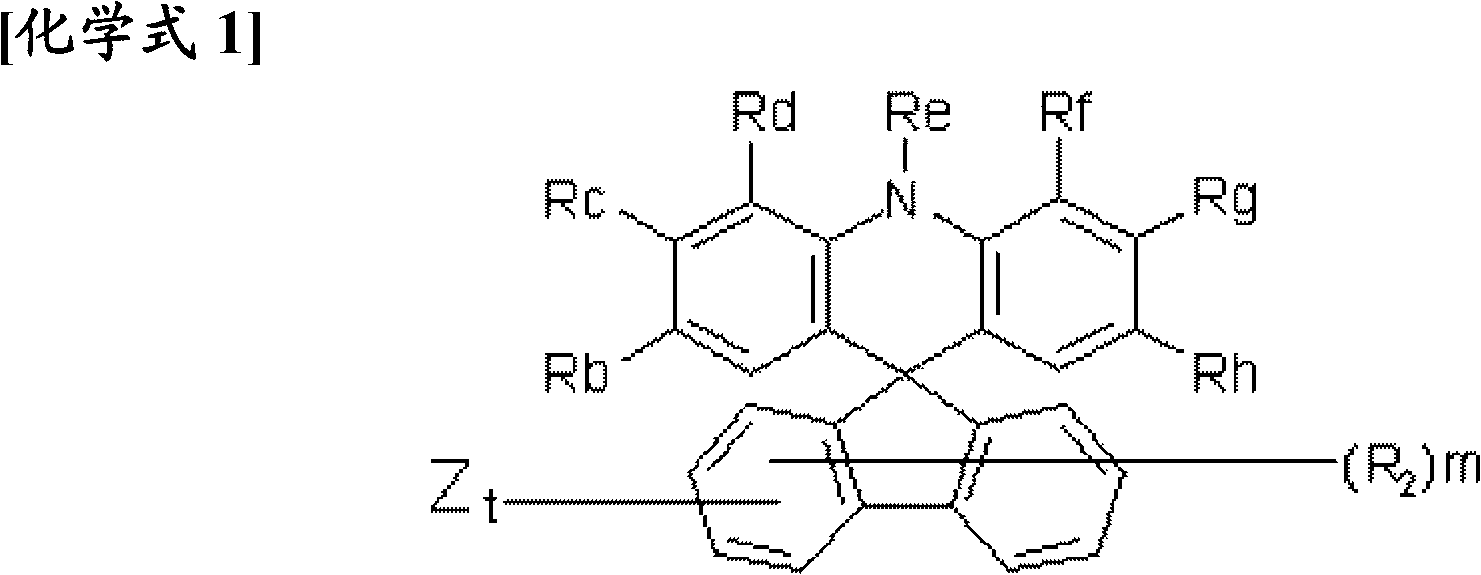
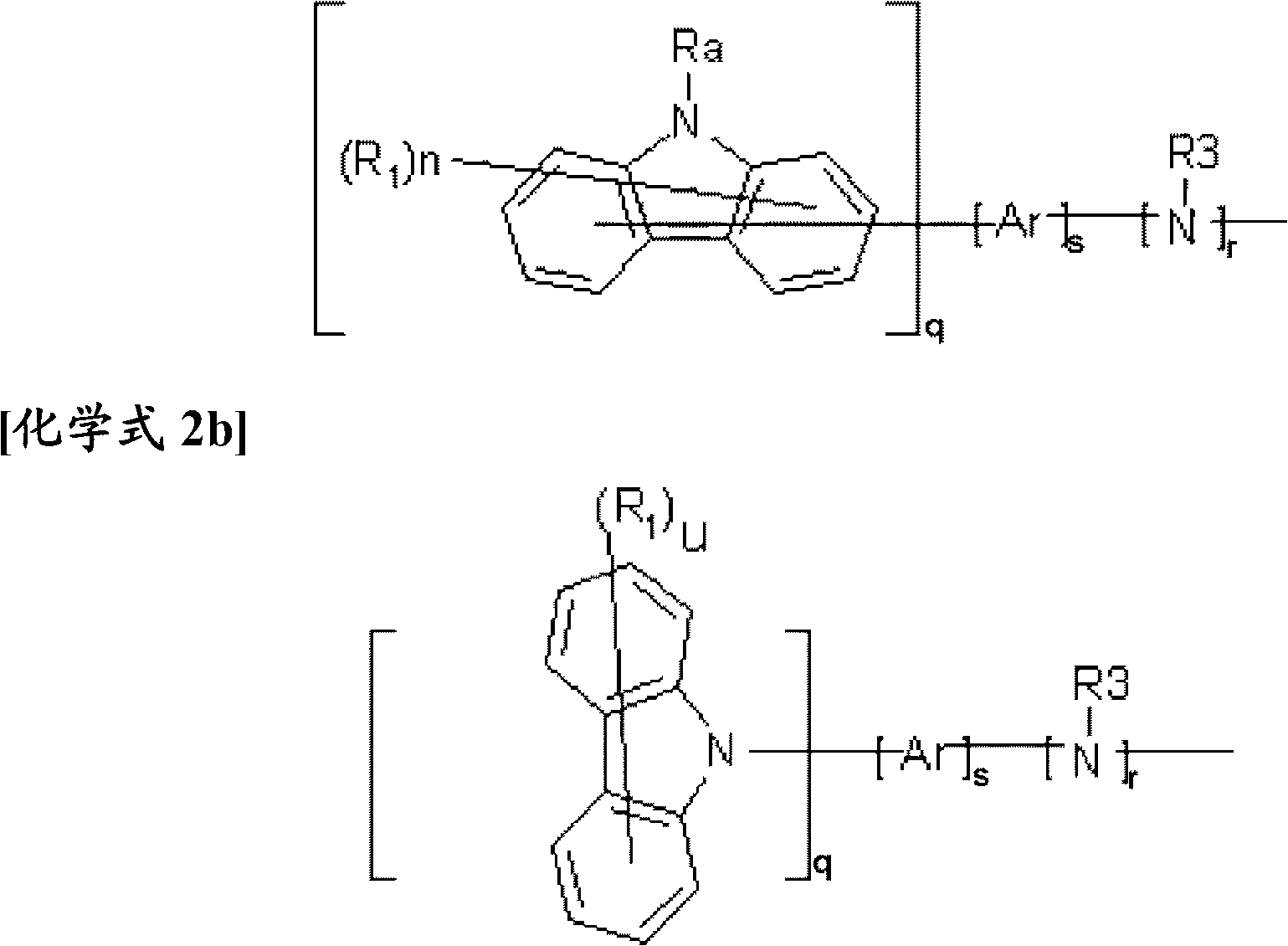
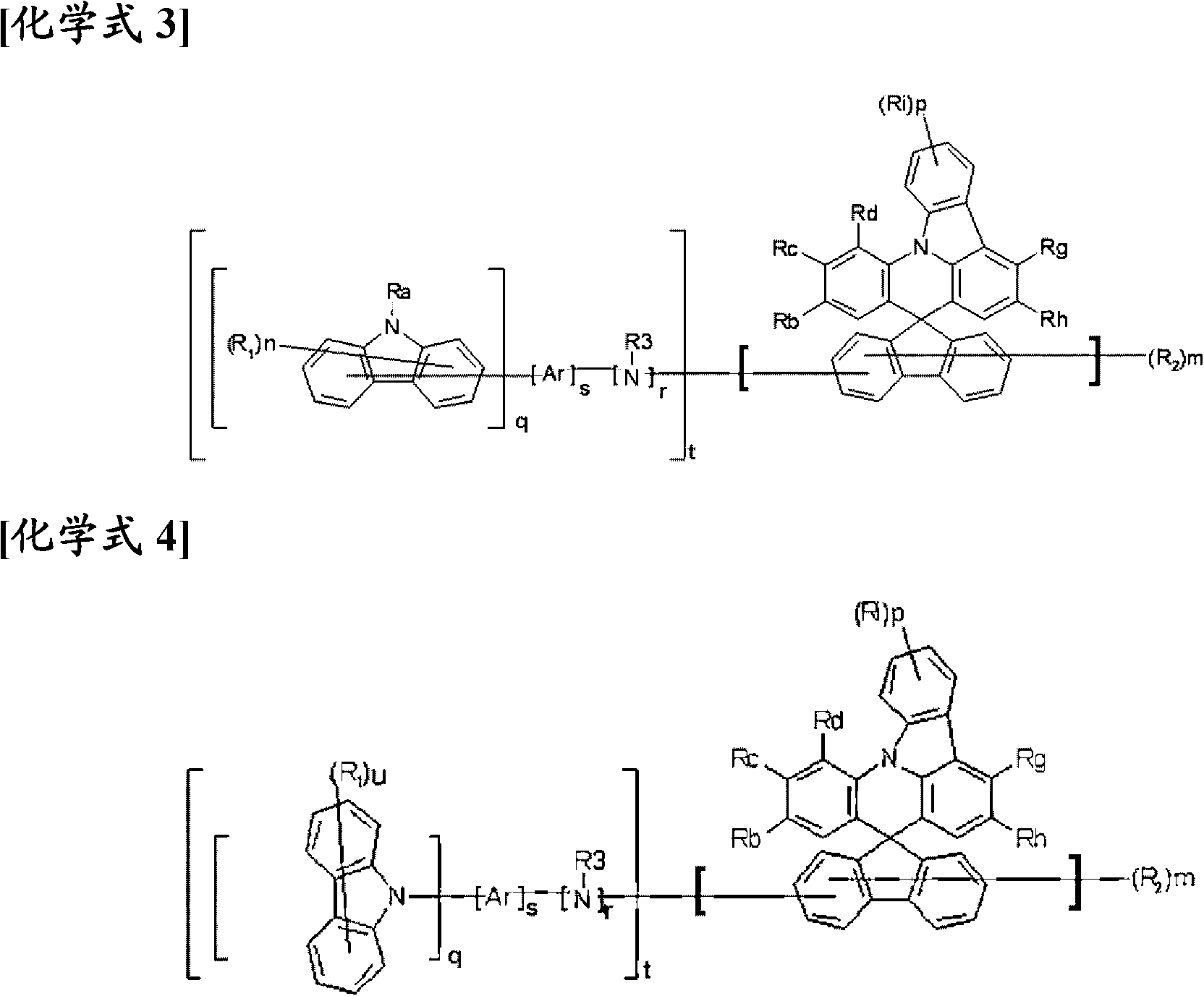
Novel compound having condensed ring, and organic electronic device using same
A compound, fused ring technology, applied in the field of organic electronic devices, can solve the problem of not developing stable and effective organic light-emitting diodes, and achieve the effect of excellent efficiency and performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation Embodiment 1
[0093] : Preparation of raw materials represented by chemical formula a
[0094] Carbazole (carbazole, 1.672g, 10mmol), 1-bromo-2-iodobenzene (1-bromo-2-iodobenzene, 1.5mL, 12mmol), potassium carbonate (K 2 CO 3 , 2.7646 g, 20 mmol), cuprous iodide (CuI, 95 mg, 0.5 mmol) and 25 mL xylene were refluxed under nitrogen atmosphere. After cooling to room temperature, the resultant was extracted with ethyl acetate and washed with anhydrous magnesium sulfate (MgSO 4 ) to remove water and remove solvent under reduced pressure. The compound was passed through a silica gel column using hexane solvent, after which the solvent was removed under reduced pressure and dried in vacuo to give the desired above compound as a white solid (800 mg, 25% yield).
[0095] MS: [M+H]+=323.
preparation Embodiment 2
[0096] : Preparation of raw materials represented by chemical formula b
[0097] After the raw material represented by chemical formula a (6.96g, 21.6mmol) was dissolved in 300ml of purified THF and cooled to -78°C, n-BuLi (2.5Min hexane, 8.64ml, 21.6mmol) was slowly added dropwise thereto. After stirring at this temperature for 30 minutes, 2,7-dibromo-9-fluorenone (2,7-dibromo-9-fluorenone, 6.08 g, 18.0 mmol) was added thereto. After stirring at this temperature for 40 minutes, the temperature was raised to normal temperature and stirring was continued for 3 hours. Ammonium chloride (NH 4 Cl) aqueous solution to complete the reaction, extracted with ether. Anhydrous magnesium sulfate (MgSO 4 ) after removing the water in the organic material layer, the organic solvent is removed. The resulting solid was dispersed in ethanol, filtered and vacuum dried after stirring for a full day to afford 10.12 g (96.7% yield) of intermediate material. After the obtained solid was dispe...
preparation Embodiment 3
[0099] : Preparation of raw materials represented by chemical formula c
[0100] Diphenylamine (1.692g, 10mmol), 1-bromo-2-iodobenzene (1-bromo-2-iodobenzene, 1.5mL, 12mmol), potassium carbonate (K 2 CO 3 , 2.7646 g, 20 mmol), cuprous iodide (CuI, 95 mg, 0.5 mmol) and 25 mL xylene were refluxed under nitrogen atmosphere. After cooling to room temperature, the product was extracted with ethyl acetate and washed with anhydrous magnesium sulfate (MgSO 4 ) to remove water and remove solvent under reduced pressure. The compound was passed through a silica gel column by using hexane solvent, after which the solvent was removed under reduced pressure and dried in vacuo to give the desired compound c above as a white solid (2.27 g, 70% yield).
[0101] MS: [M+H]+=324.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com