Method for preparing titanium dioxide nanometer material with exposed nonmetal-metal co-doped (001) surface
A technology of nanomaterials and metal doping, applied in chemical instruments and methods, chemical/physical processes, physical/chemical process catalysts, etc., to achieve the effects of improved transfer efficiency, easy control of the preparation process, and improved degradation efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] 1) Add 1 g of titanium dioxide, 0.1 g of ferric nitrate, 0.1 g of ammonium fluoride and 40 mL of 5 mol / L sodium hydroxide solution into the hydrothermal reaction kettle and stir well. Then it was hydrothermally reacted at 110 °C for 24 hours, and then the reaction product was acid-washed, washed with water, and dried to obtain N+Fe co-doped H 2 Ti 3 o 7 nanomaterials. ,
[0032] 2) Take 1 g N+Fe co-doped H 2 Ti 3 o 7 Nanomaterials, 0.6 ml of hydrofluoric acid with a concentration of 0.96mol / L was added to the hydrothermal reaction kettle, and the hydrothermal reaction was carried out at 150 °C for 10 hours, and then the reaction products were washed with water and dried to obtain +Fe co-doped (001) facet exposed TiO2 nanomaterials.
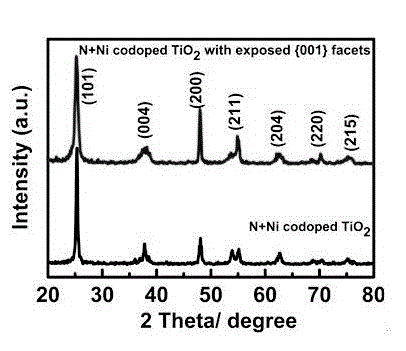
[0033] figure 1 Shown is the N+Fe co-doped (001) surface exposed TiO prepared in Example 1 2 Anatase phase TiO prepared by nanomaterials and comparative examples 2 The X-ray diffraction (XRD) comparison chart of nanoparticles, as...
Embodiment 2
[0035]1) Take 5 g of titanium dioxide, 5 g of chromium chloride, 5 g of ammonium fluoride and 40 mL of 10 mol / L sodium hydroxide solution into the hydrothermal reaction kettle and stir well. Then it was subjected to a hydrothermal reaction at a temperature of 150 °C for 48 hours, and then the reaction product was acid-washed, washed with water, and dried in sequence to obtain N+Cr co-doped H 2 Ti 3 o 7 nanomaterials. ,
[0036] 2) Take 1 g N+Cr co-doped H 2 Ti 3 o 7 Nanomaterials, 1.2 ml of hydrofluoric acid with a concentration of 1.92 mol / L was added to the hydrothermal reaction kettle, and the hydrothermal reaction was carried out at 200 ° C for 18 hours, and then the reaction product was washed with water in sequence and dried to obtain N+Cr co-doped (001) facet exposed TiO2 nanomaterials.
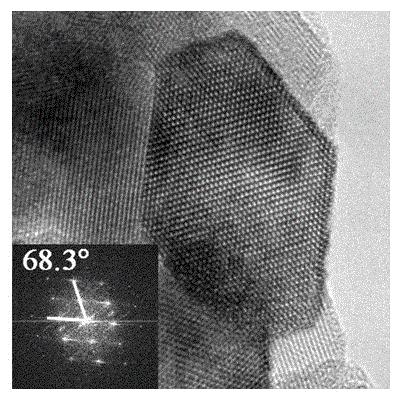
[0037] figure 2 Shown is the N+Cr co-doped (001) surface exposed TiO prepared in Example 2 2 High-resolution transmission electron microscope (HRTEM) image of nanomaterials. ...
Embodiment 3
[0039] 1) Take 10 g of titanium dioxide, 1 g of nickel nitrate, 1 g of ammonium fluoride and 40 mL of 15 mol / L sodium hydroxide solution into the hydrothermal reaction kettle and stir well. Then it was subjected to hydrothermal reaction at 130 °C for 72 hours, and then the reaction product was acid-washed, washed with water, and dried in sequence to obtain N+Ni co-doped H 2 Ti 3 o 7 nanomaterials. ,
[0040] 2) Take 1 g N+Ni co-doped H 2 Ti 3 o 7 Nanomaterials, 2.4 ml of hydrofluoric acid with a concentration of 3.84mol / L was added to the hydrothermal reaction kettle, and the hydrothermal reaction was carried out at 180 °C for 24 hours, and then the reaction product was washed with water and dried to obtain +Ni co-doped (001) facet exposed TiO2 nanomaterials.
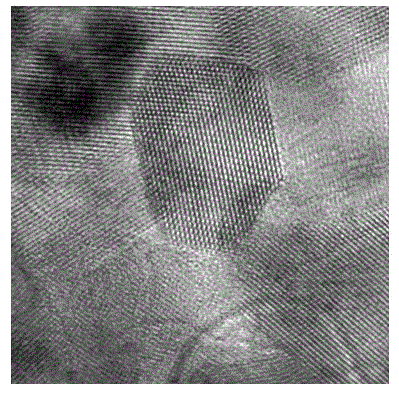
[0041] image 3 Shown is the high-resolution transmission electron microscope (HRTEM) image of the N+Ni co-doped (001) surface exposed TiO2 nanomaterial prepared in Example 3. It can be seen from the figure that...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com