Brookite titanium dioxide nanocrystalline and preparation method and application thereof
A titanium dioxide, nanocrystal technology, applied in the direction of titanium dioxide, chemical instruments and methods, titanium oxide/hydroxide, etc., can solve the limitation of large-scale production and application, the difficulty of pure-phase brookite synthesis, the difficulty of brookite synthesis, etc. problem, to achieve the effects of excellent catalytic activity, low production cost, and easy variation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
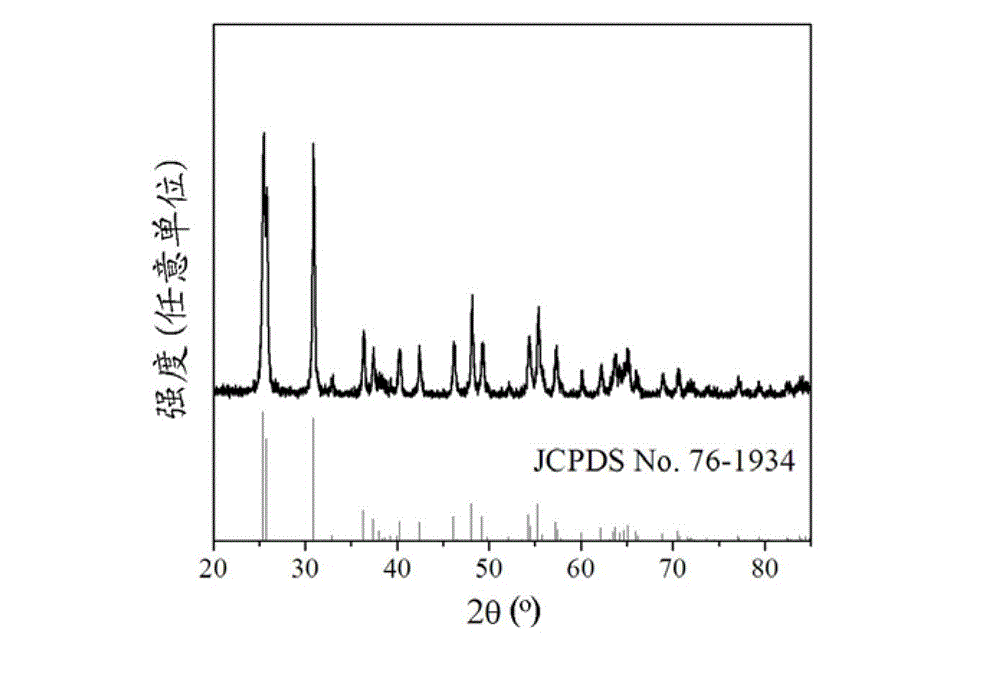
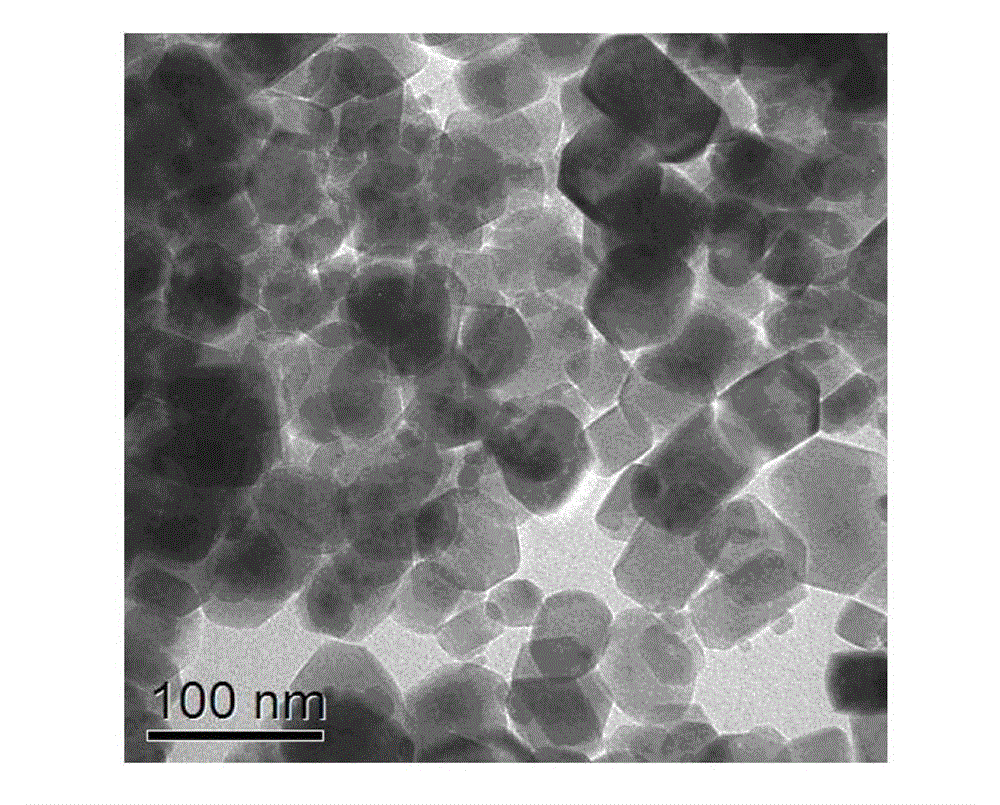
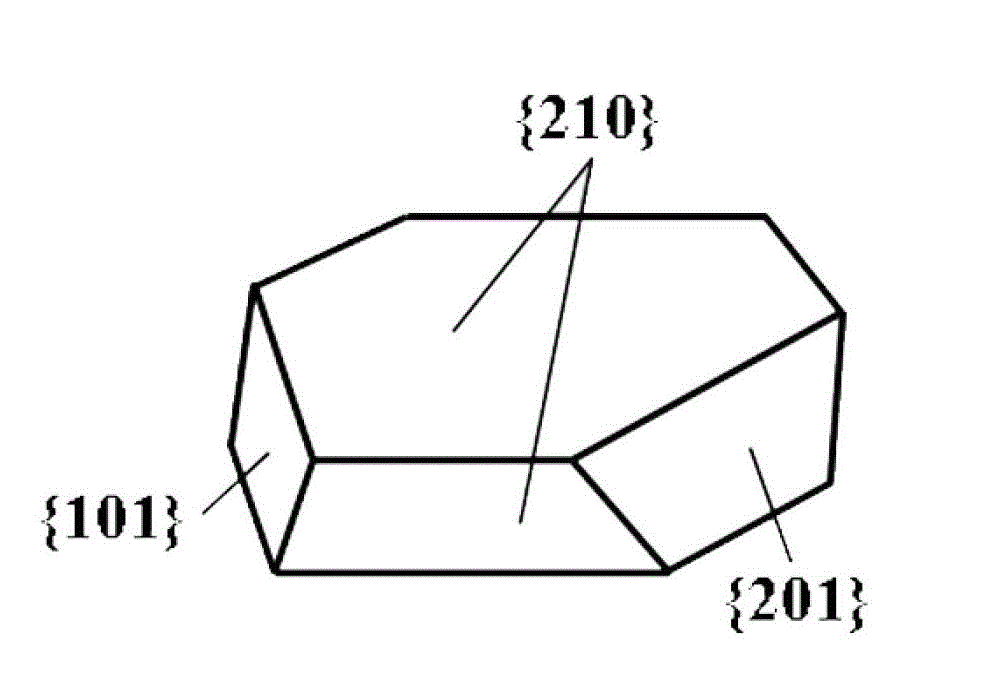
[0030] Example 1: In a fume hood, TiCl 4 The liquid was slowly added dropwise into the deionized water cooled and stirred in an ice-water bath, and the concentration of the solution was adjusted to 0.5 M. The above titanium-containing solution was taken out from the ice-water bath, and stirred at room temperature for about 60 min. Then urea was added to the above solution and stirred to dissolve, and its concentration was controlled to be 1.0 M. Next, sodium lactate solution (50%) was added to the mixed solution and stirred evenly so that the concentration of sodium lactate was maintained at 0.4 M. Finally, the mixed solution is packed into the reactor and sealed, 190 o C for 12 h. After the reaction was completed, the product was separated, washed 3 times by centrifugation with ethanol and deionized water, and placed in an oven at 90 o C drying for 12 h to obtain a white powder sample. The XRD patterns of the prepared brookite samples are as follows figure 1 As shown, t...
Embodiment 2
[0031] Example 2: In a fume hood, TiCl 4 The liquid was slowly added dropwise into the deionized water cooled and stirred in an ice-water bath, and the concentration of the solution was adjusted to 0.3 M. The above titanium-containing solution was taken out from the ice-water bath, and stirred at room temperature for about 30 min. Then urea was added to the above solution and stirred to dissolve, and its concentration was controlled to be 0.8 M. Next, sodium lactate solution (50%) was added to the mixed solution and stirred evenly so that the concentration of sodium lactate was maintained at 0.2 M. Finally, the mixed solution is packed into the reactor and sealed, 200 o C for 12 h. After the reaction was completed, the product was separated, washed 3 times by centrifugation with ethanol and deionized water, and placed in an oven at 80 o C drying for 10 h to obtain a white powder sample.
Embodiment 3
[0032] Example 3: In a fume hood, TiCl 4 The liquid was slowly added dropwise into the deionized water cooled and stirred in an ice-water bath, and the concentration of the solution was adjusted to 0.5 M. The above titanium-containing solution was taken out from the ice-water bath, and stirred at room temperature for about 60 min. Then urea was added to the above solution and stirred to dissolve, and its concentration was controlled to be 1.2 M. Next, sodium lactate solution (50%) was added to the mixed solution and stirred evenly so that the concentration of sodium lactate was maintained at 0.3 M. Finally, the mixed solution is packed into the reactor and sealed, 190 o C reacted for 20 h. After the reaction was completed, the product was separated, washed 3 times by centrifugation with ethanol and deionized water, and placed in an oven at 90 o C drying for 12 h to obtain a white powder sample.
[0033] (2) Preparation of precious metal-supported brookite titanium dioxide...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com