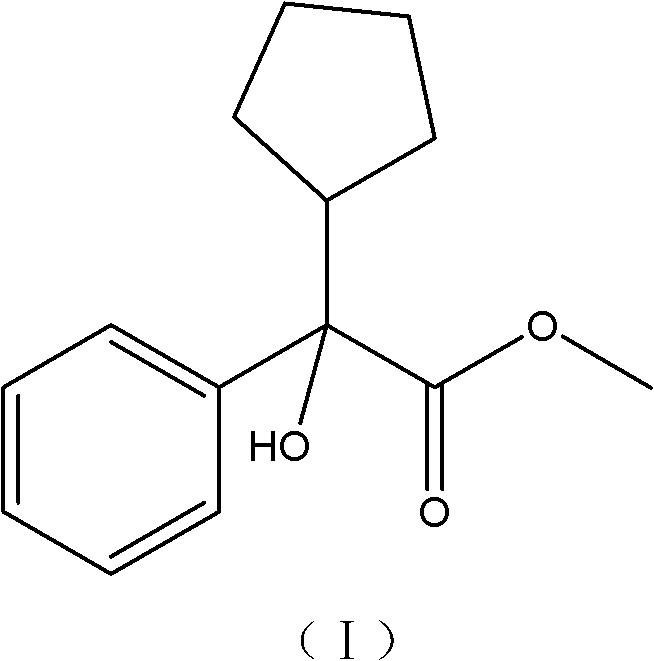
Preparation method of a-cyclopentyl methyl mandelate
A technology of methyl cyclopentylmandelate and methyl ester, which is applied in the field of preparation of the intermediate a-cyclopentylmandelic acid methyl ester, and can solve problems such as unsafe use, high cost, and low yield of cyclopentadiene , to achieve the effect of convenient operation, simple steps and high safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0026] The method for preparing methyl α-cyclopentylmandelic acid provided by the present invention, such as figure 1 Shown, including:
[0027] (1) Preparation of a-chlorocyclopentane Grignard reagent: magnesium chips and chlorocyclopentane (C 5 H 9 Cl) react in an organic solvent such as anhydrous ether or tetrahydrofuran to generate a-chlorocyclopentane Grignard reagent;
[0028] (2) Preparation of crude a-cyclopentyl mandelic acid methyl ester: firstly, methyl benzoyl formate (C 9 H 8 O 3 ) Dissolve in an organic solvent such as anhydrous ether or tetrahydrofuran to form a methyl benzoyl formate solution, and react the Grignard reagent of a-chlorocyclopentane generated in step (1) with the methyl benzoyl formate solution Generate crude a-cyclopentyl mandelic acid methyl ester (C 14 H 18 O 3 );
[0029] (3) Purification of the crude methyl a-cyclopentylmandelate: the crude methyl a-cyclopentylmandelate obtained in step (2) is hydrolyzed and esterified to obtain pure methyl a-cycl...
Embodiment 1
[0034] Step 1 is the preparation of a-chlorocyclopentane Grignard reagent, specifically:
[0035] Weigh 146g of magnesium scraps and add it to 1000ml of anhydrous ether (which can be replaced by tetrahydrofuran). Under the protection of nitrogen, first add 90g of chlorocyclopentane dropwise, stir to initiate the reaction, and continue to add 600g of chlorine after the initiation is successful. The reaction of cyclopentane to produce a-chlorocyclopentane is a Grignard reagent. After the initiation is successful, the dripping process should keep the reaction liquid slightly boiling, and then stir for 1 hour after the dripping is completed, for use;
[0036] Step 2 is the preparation of crude a-cyclopentyl mandelic acid methyl ester, specifically:
[0037] Dissolve 500g of methyl benzoyl formate in 1200ml of anhydrous ether, dissolve methyl benzoyl formate in ether or tetrahydrofuran to form a solution of methyl benzoyl formate, and replace the a-chloro ring generated in step 1. The Gr...
Embodiment 2
[0043] Step 1 is the preparation of a-chlorocyclopentane Grignard reagent, specifically:
[0044] Weigh 1.5kg of magnesium scraps and add it to 8L of anhydrous ether (which can be replaced by tetrahydrofuran), under the protection of nitrogen, first add 0.9kg of chlorocyclopentane dropwise, let the reaction start under stirring, and continue to add 6.2kg of chlorine after the initiation is successful Substitute cyclopentane, the dropping rate is maintained at a rate that makes the reaction liquid slightly boiling, and after the dropping is completed, stir for another 2 hours for standby.
[0045] Step 2. Step 2 is the preparation of crude a-cyclopentyl mandelic acid methyl ester, specifically:
[0046] Dissolve 5.2 kg of methyl benzoyl formate in 15 L of anhydrous ether to form a solution of methyl benzoyl formate. Add the a-chlorocyclopentane Grignard reagent in the ether solution prepared in step 1 dropwise to the benzene In the methyl acylformate solution, keep the temperature at...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com