Preparation method of bismuth vanadate visible light photocatalysis material
A photocatalytic material, bismuth vanadate technology, applied in the field of environmental pollution control, can solve the problem of low catalytic efficiency of visible light, achieve good degradation effect, easy to obtain and cheap raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] The preparation method of the bismuth vanadate of the present embodiment may further comprise the steps:
[0027] 1) Take Bi(NO 3 ) 3 ·5H 2 O (97%), NH 4 VO 3 (98.5%) as the source material and citric acid (99.5%) as the chelating agent. Weigh 0.01 mol, 4.8580 g of Bi(NO 3 ) 3 ·5H 2 O and 0.02 mol, 4.2028 g of citric acid monohydrate (C 6 h 8 o 7 ·H 2 O), add citric acid monohydrate to 50mL Bi(NO 3 ) 3 ·5H 2 O solution, A solution was obtained. Weigh 0.01 mol, 1.1698 g of NH at a molar ratio of 1:2 4 VO 3 and 0.02 mol, 4.2028 g of citric acid monohydrate (C 6 h 8 o 7 ·H 2 O), solution B dissolved in 50 mL of boiling distilled water. Mix liquid A and liquid B according to the molar ratio of Bi:V=1:1, adjust the pH value to about 6.5 with ammonia water, keep stirring at 80 ℃, evaporate, and finally obtain the dark blue bismuth vanadate precursor sol.
[0028] 2) The dried bismuth vanadate precursor sol was put into a muffle furnace and calcined at 500°...
Embodiment 2
[0033] With Bi(NO 3 ) 3 ·5H 2 O (97%), NH 4 VO 3 (98.5%) as the source material and citric acid (99.5%) as the chelating agent. Weigh 0.01 mol, 4.8580 g of Bi(NO 3 ) 3 ·5H 2 O and 0.02 mol, 4.2028 g of citric acid monohydrate (C 6 h 8 o 7 ·H 2 O), add citric acid monohydrate to 50mL Bi(NO 3 ) 3 ·5H 2 O solution, A solution was obtained. Weigh 0.01 mol, 1.1698 g of NH at a molar ratio of 1:2 4 VO 3 and 0.02 mol, 4.2028 g of citric acid monohydrate (C 6 h 8 o 7 ·H 2 O), solution B dissolved in 50 mL of boiling distilled water. Mix liquid A and liquid B according to Bi:V=1:1 molar ratio, according to Eu 3+ with Bi 3+ The molar ratio is 0.1% adding Eu(NO 3 ) 3 In the mixed solution, adjust the pH value to about 6.5 with ammonia water, continue to stir at 80 ° C, evaporate, and finally obtain a dark blue bismuth vanadate precursor sol.
[0034] 2) Put the dried bismuth vanadate precursor sol into a muffle furnace and calcinate at 500°C for 5 hours to obtain...
Embodiment 3
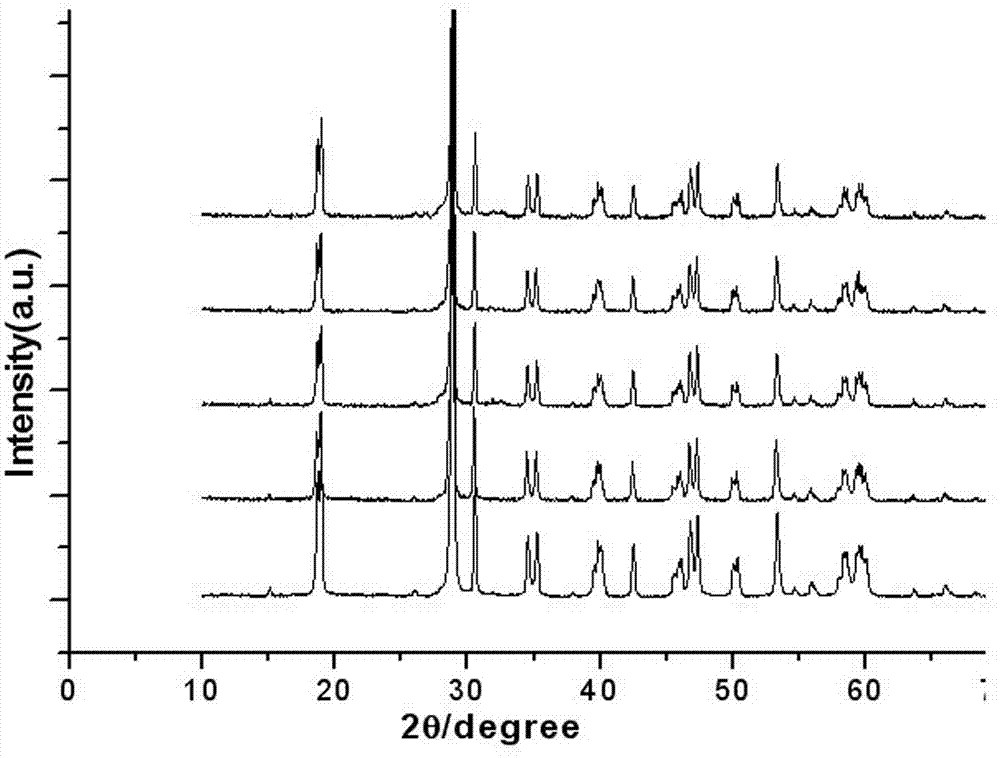
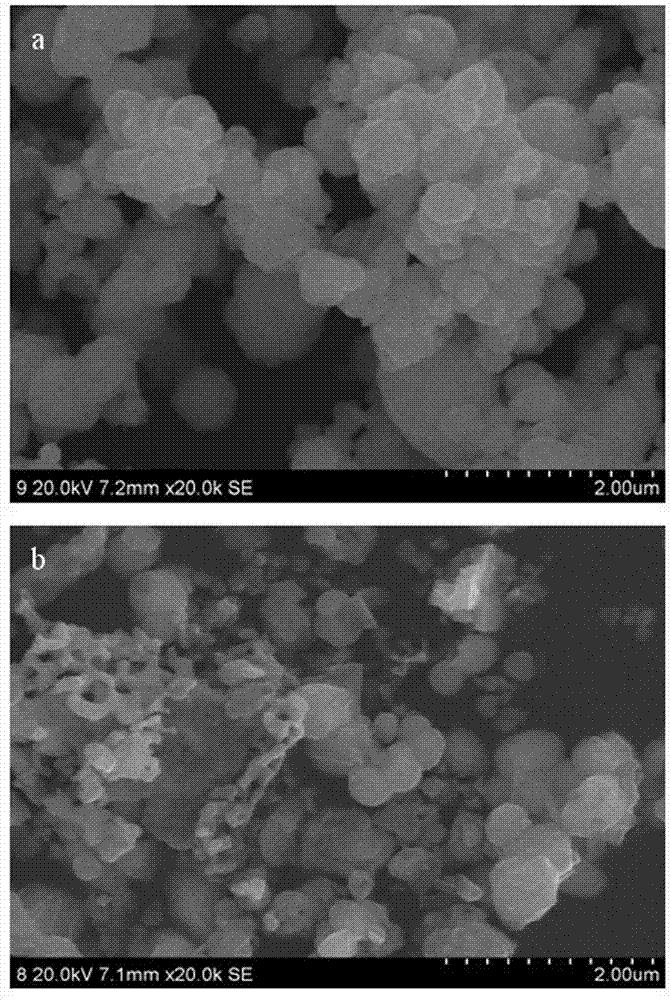
[0038] According to the preparation method of the present invention of embodiment 2, only the Eu in embodiment 2 3+ with Bi 3+ The molar ratio was changed to 0.2%, and 0.1% Eu was prepared 3+ Doped bismuth vanadate photocatalytic material. The UV-vis spectrum of the product obtained is shown in figure 1 (c), BiVO synthesized in this example 4 The optical absorption threshold of 556nm, the forbidden band width is 2.19 eV. figure 2 (c) is the XRD pattern of the product, the BiVO synthesized in this example 4 at 18.7 0 , 28.8 0 , 30.5 0 , 34.5 0 , 35.1 0 , 37.8 0 , 39.8 0 , 42.4 0 , 47.2 0 , 53.2 0 , 59.8 0 , 63.6 0 , 69.3 0 The diffraction peak at the position is the characteristic peak of monoclinic bismuth vanadate, but the characteristic peak is obviously shifted to a small angle, and the unit cell volume is 310.85 nm 3 . image 3 (b) is the SEM image of the product. It can be seen from the image that some particles in the generated product sample are re...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| catalytic efficiency | aaaaa | aaaaa |
| catalytic efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com