Preparation method of alpha-fluoroacrylate
A technology of fluoroacrylate and fluoroacetate is applied in the field of preparation of α-fluoroacrylate, can solve the problems of low purity, low yield of α-fluoroacrylate, etc. high rate effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
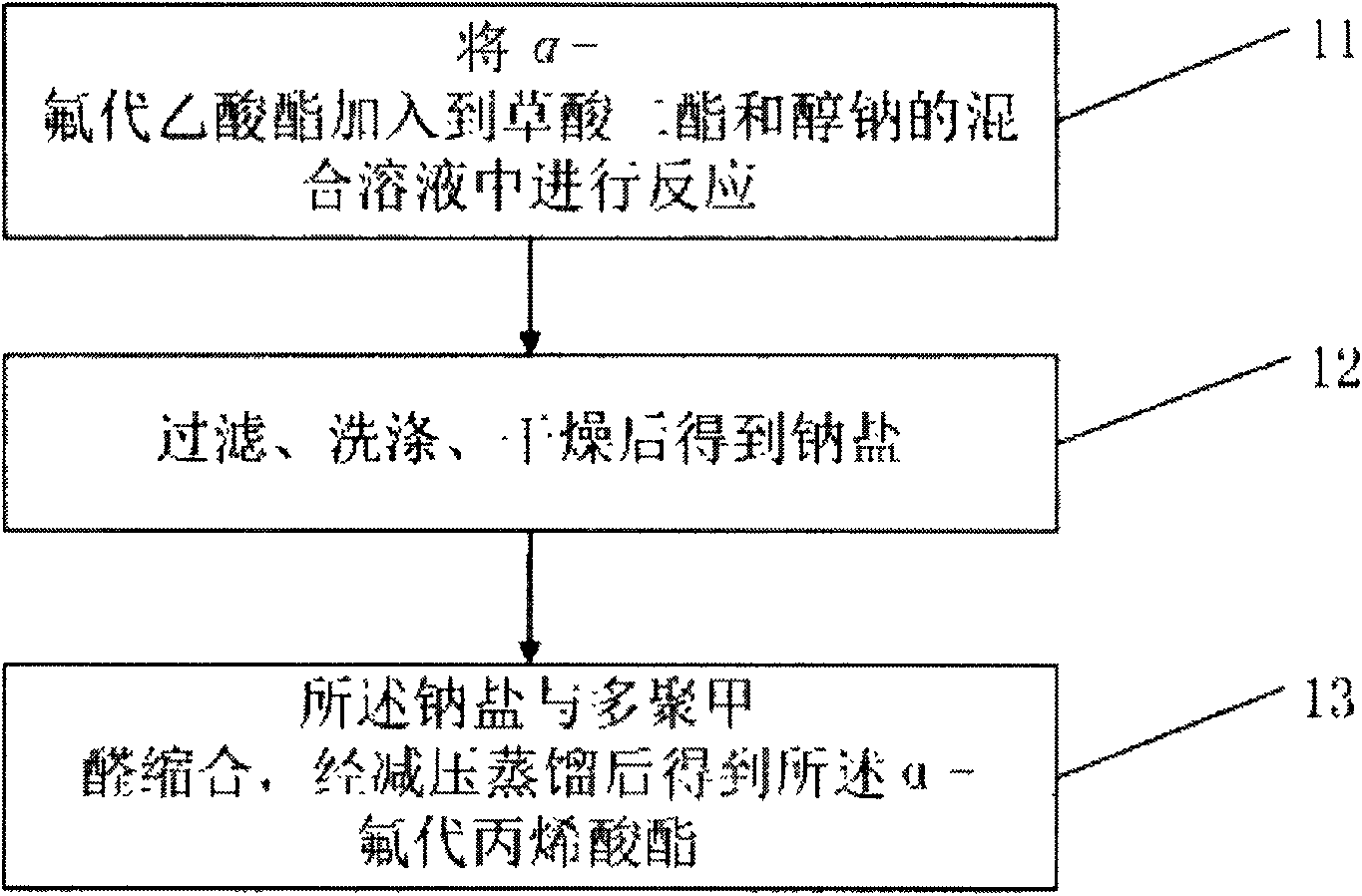
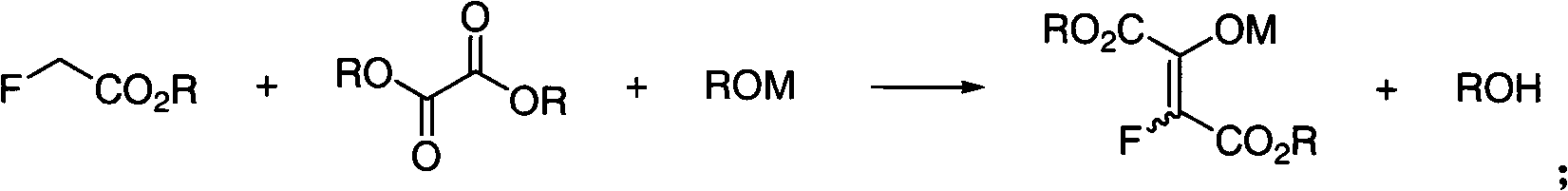
[0025] First, please refer to figure 1 , figure 1 It is a flow chart of the preparation method of a kind of α-fluoroacrylate of the present invention, from figure 1 It can be seen that the present invention comprises the following steps: Step 11: adding α-fluoroacetate to the mixed solution of oxalic acid diester and sodium alkoxide for reaction; the chemical reaction formula is:
[0026]
[0027] Step 12: Sodium salt is obtained after filtration, washing and drying
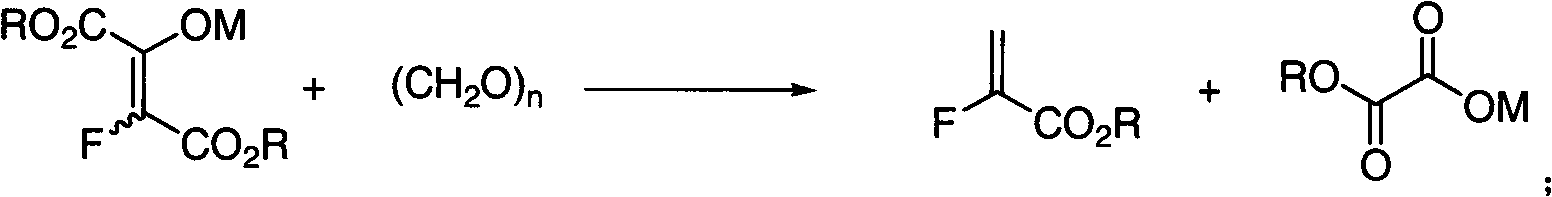
[0028] Step 13: The sodium salt is condensed with paraformaldehyde, and the α-fluoroacrylate is obtained after vacuum distillation. The chemical reaction formula is:
[0029]
[0030] R in the above two chemical reaction formulas is a C1-C8 alkyl group, and M is an alkaline earth metal ion such as sodium, potassium, lithium or the like. The alkyl group includes methyl, ethyl, n-propyl, isopropyl, n-butyl, isobutyl, n-pentyl, isopentyl, n-hexyl or isohexyl; alkaline earth metal ions include sodium, potass...
example 1
[0034] Example 1: Preparation of dimethyl sodium 2-fluoro-3-oxosuccinate
[0035] On the dried four-necked flask (500mL), add a condenser tube, a drying tube (anhydrous CaCl 2 ), thermometer, mechanical stirring, constant pressure dropping funnel. Sodium methoxide (56 g), freshly distilled dry methyl tert-butyl ether (dried over 4A molecular sieves) (400 mL), and dimethyl oxalate (118.09 g) were first added. The temperature of the external bath is controlled at about 30°C, and stirred for 30 minutes. Then methyl fluoroacetate (92.07 g) was added dropwise through a constant pressure dropping funnel to control the reaction at 30° C. (about 40 min). Stirring was continued at 20-30 °C until the reaction was complete (about 14 h). A large amount of light-colored finely divided powdery solid appeared in the reaction flask. The reaction liquid was filtered, and the filter cake was washed with anhydrous methyl tert-butyl ether (50 mL*3) until the washing liquid was colorless. Aft...
example 2
[0036] Example 2: Preparation of α-methyl fluoroacrylate
[0037] On the dried two-necked flask (500mL), add a 40mm glass sleeve, an exhaust joint, a thermometer, and a magnet. Add sodium dimethyl 2-fluoro-3-oxosuccinate (40 g) and dimethyl sulfoxide (300 mL) (dried over 4A molecular sieves), stir for 20 min, and then add paraformaldehyde (6 g). The temperature of the reaction solution was raised to 40° C., and the reaction was completed after about 1.5 h. Connect the vacuum distillation device, and carry out vacuum distillation operation [25mmHg / 60 ℃ (external bath temperature)]. Crude methyl α-fluoroacrylate was obtained. The crude product was washed with 60 mL of saturated saline solution. The upper organic phase was dried over anhydrous sodium sulfate. 19 g of methyl α-fluoroacrylate was obtained by filtration with a purity of >98%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com