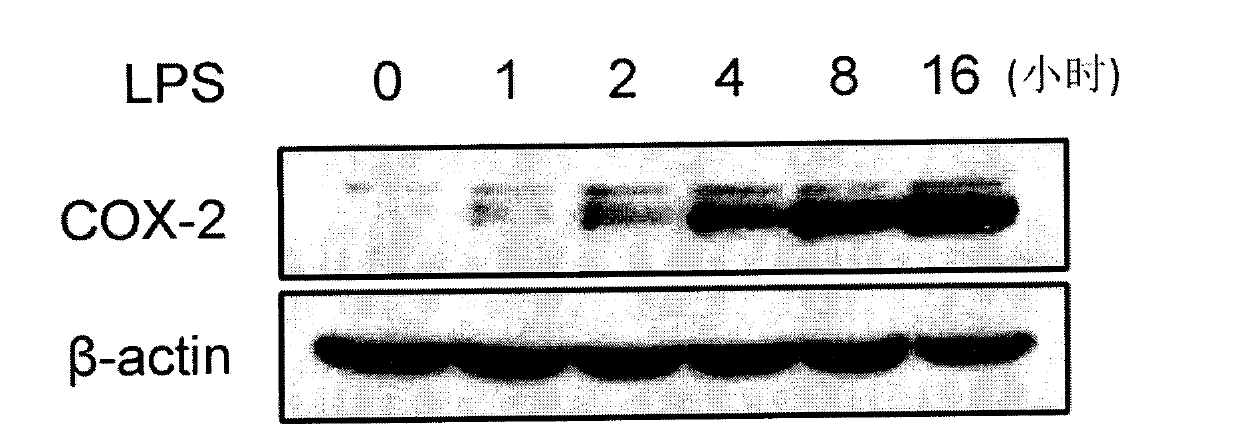
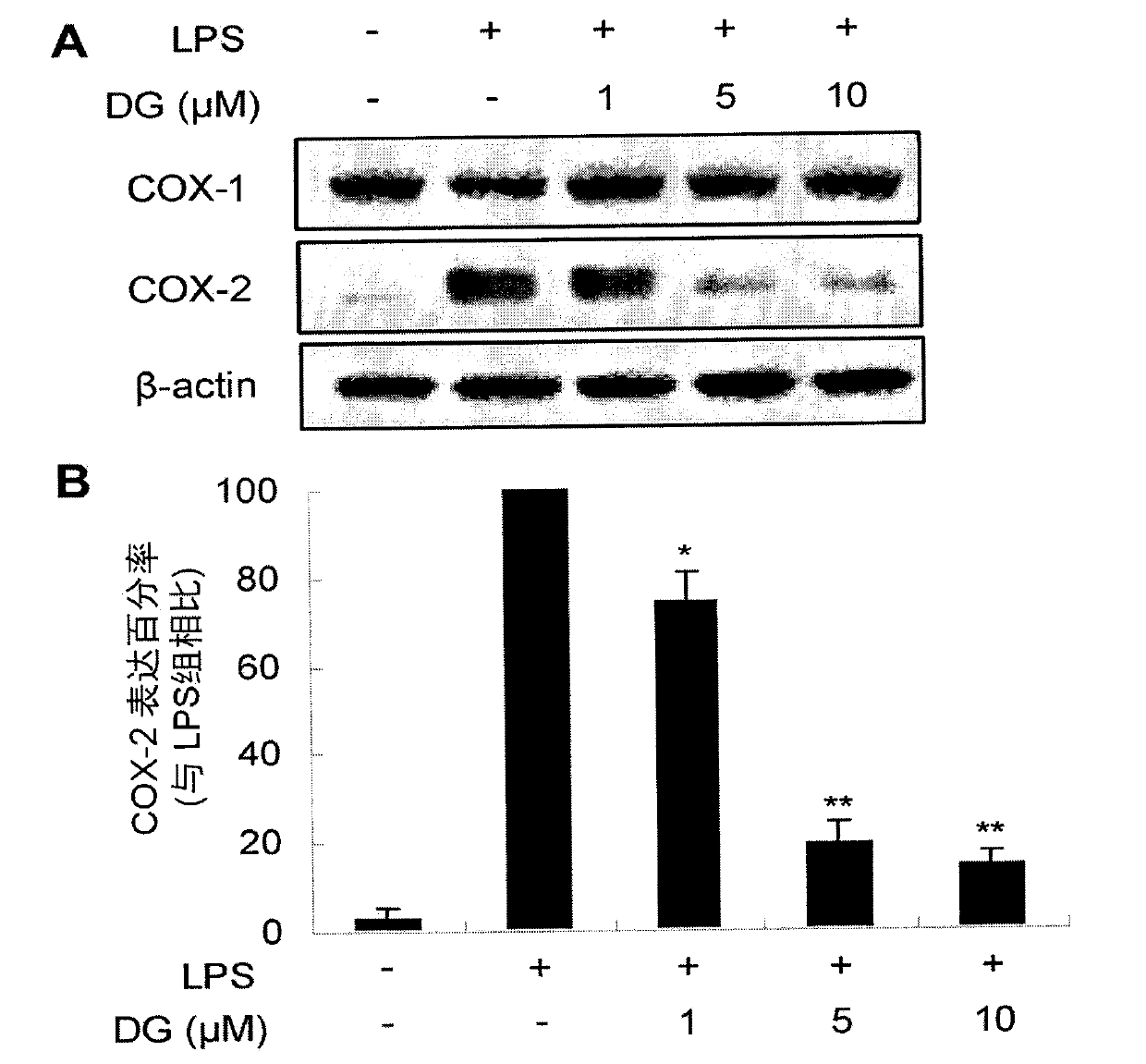
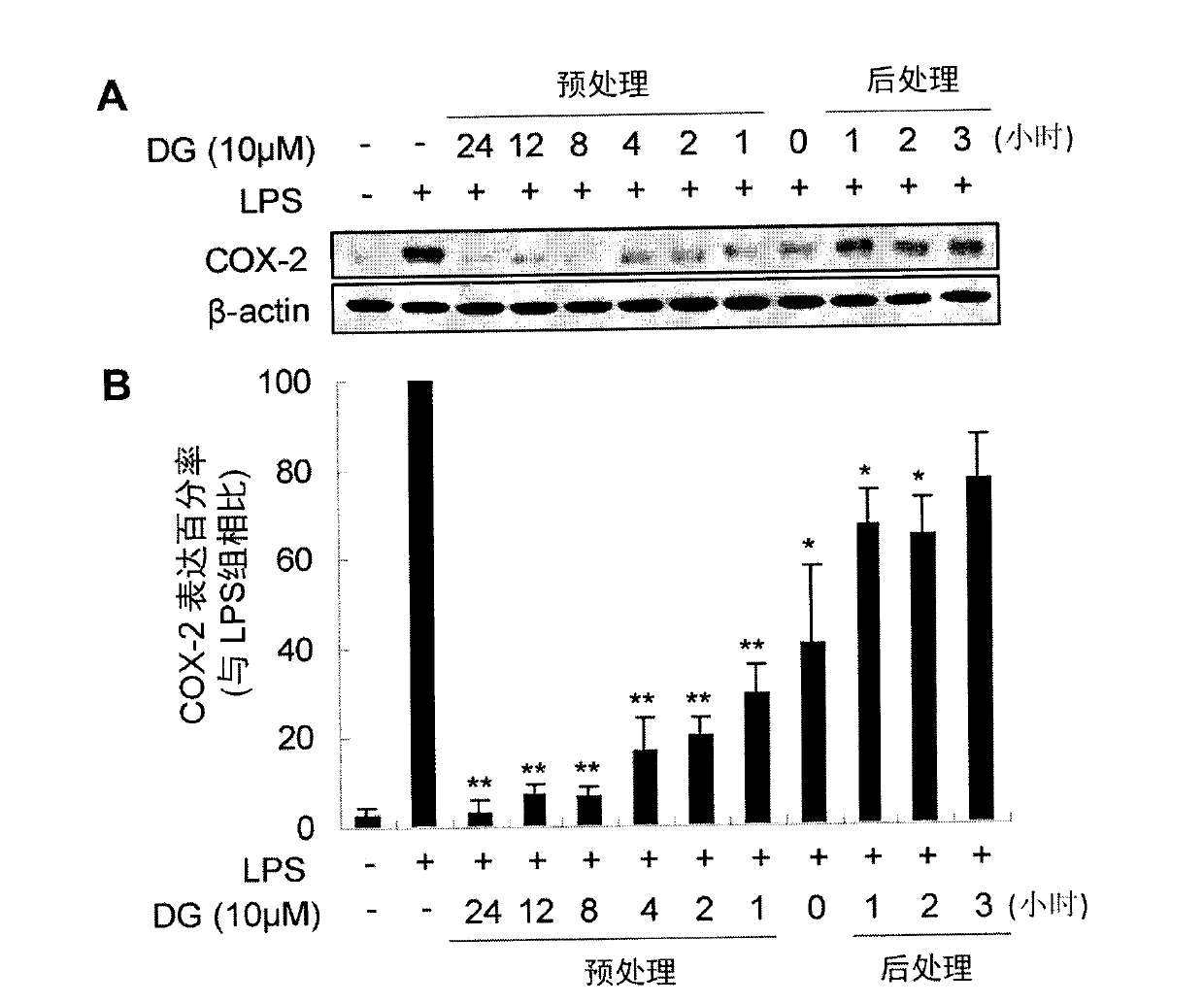
Paeoniflorin compound with inhibitory activity against abnormal expression of cyclooxygenase-2, its preparation method and application
A compound, the technology of paeoniflorin, applied in the field of medicinal chemistry, can solve the problems of not showing activity and limiting the medicinal properties of paeoniflorin
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment
[0075] 1. Instruments and equipment
[0076] NOVOstar multifunctional microplate reader (BMG LABTECH, Germany);
[0077] Electrophoresis apparatus and semi-dry electroblotting tank (Bio-Rad Laboratories, Hercules, CA);
[0078] PCR machine (MJ Research, South San Francisco, CA);
[0079] Gel Imager (Furi Technology Company);
[0080] Olympus IX51 research grade inverted fluorescence microscope (Olympus, Japan);
[0081] Infrared spectrum: Perkin-Elmer 577 infrared spectrophotometer;
[0082] Mass spectrometer: Finnigan MAT-95, Finnigan LCQ-DECA mass spectrometer (low resolution ESI).
[0083] Nuclear magnetic resonance spectrum: Varian Mecury Plus-300 nuclear magnetic resonance instrument, Bruker AM-300 nuclear magnetic resonance instrument, δ (ppm), with TMS as internal standard;
[0084] LC-MS: Agilent 1100 liquid phase coupled bruker esquire mass spectrometer;
[0085] Analytical HPLC instrument: Waters 2690 Separate Model, Waters PDA 996 detector coupled with Alltch ...
Embodiment 1
[0098] Embodiment 1 compound DG synthetic method
Embodiment 11
[0100]
[0101] Paeoniflorin (1) (4.8g, 10mmol) was dissolved in DMF (30mL), TBSCl (1.5g, 10mmol) and imidazole (1.4g, 20mmol) were added, stirred at room temperature for 1 hour, and the reaction was monitored by TLC. After the reaction was complete, water was added, extracted with ethyl acetate, and the ester layer was washed with a saturated NaCl aqueous solution, dried over anhydrous magnesium sulfate, filtered, and concentrated. The residue was purified by silica gel column chromatography (dichloromethane / methanol=15 / 1 (V / V)) to obtain 5.5 g of Compound 2 as a colorless oil. Yield: 93%.
[0102] Compound 2: ESI-MS: [M+Na]+: 617. 1 H-NMR (CDCl 3 , 300Hz, δppm): 8.18-7.83(m, 2H), 7.54(m, 1H), 7.48-7.31(m, 2H), 5.51(s, 1H), 4.82-4.35(m, 3H), 3.94-3.16 (m, 5H), 2.59(m, 1H), 2.37(m, 1H), 2.24-1.74(m, 3H), 1.34(s, 3H), 0.87(9H, s), 0.04(3H, s), 0.03(3H,s).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com