Anionic type gold complex and application thereof
An anionic and gold complex technology, applied in the direction of gold organic compounds, can solve the problems of limited conductivity and water solubility, and achieve the effects of good stability, safety and reliability, and high product purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
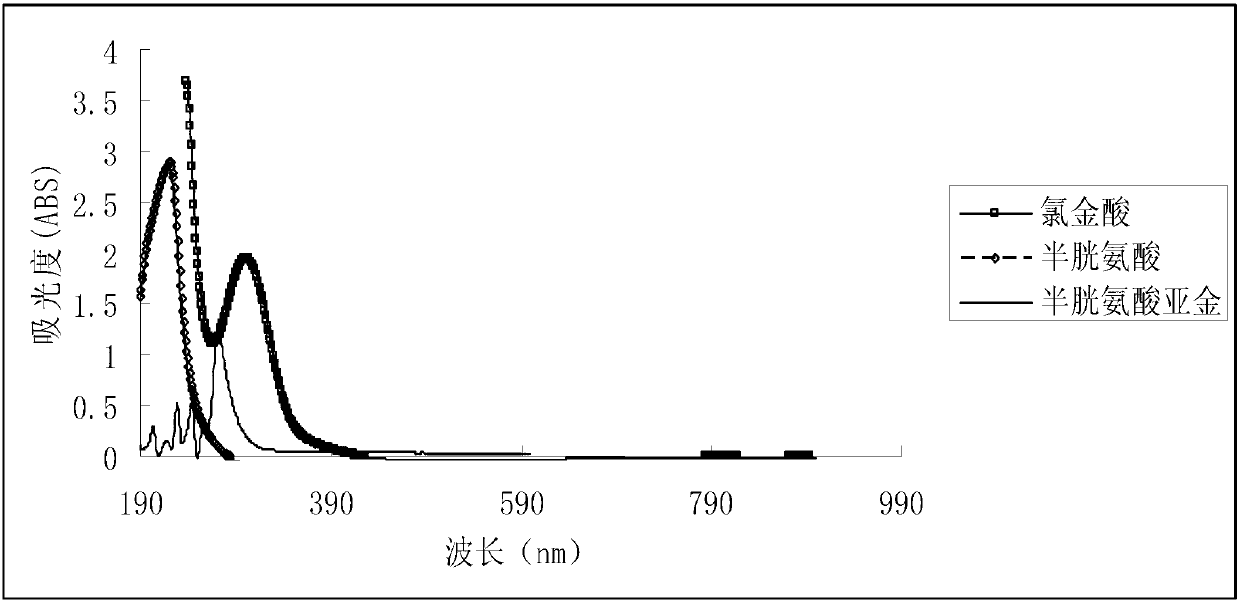
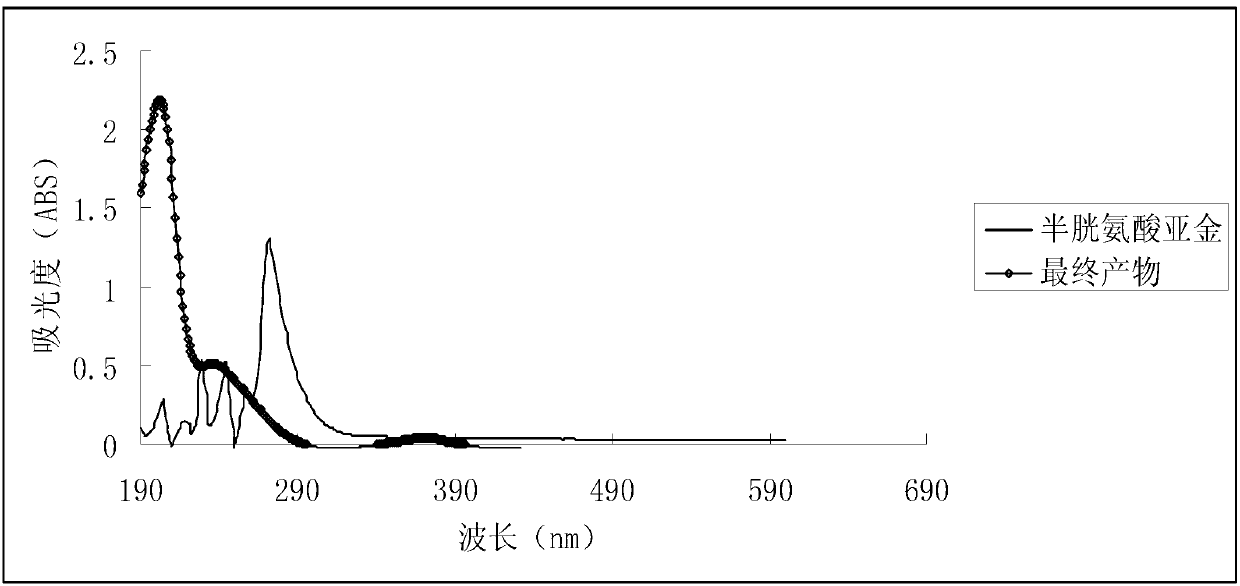
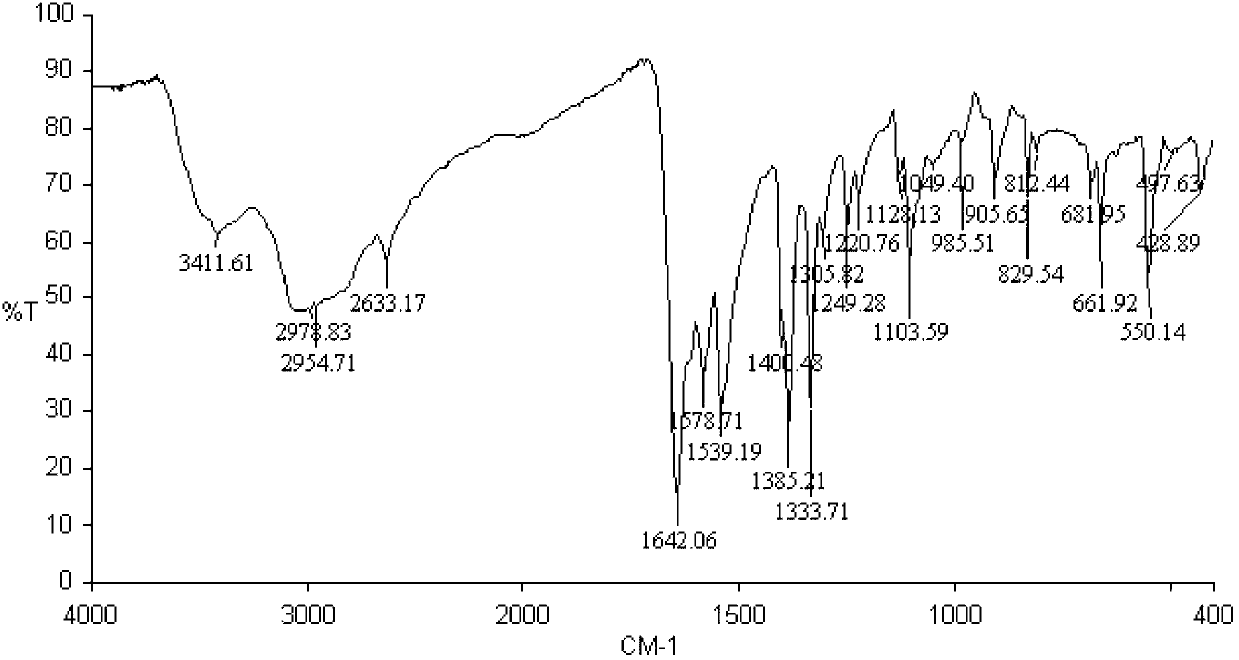
[0030] Take 2.35 grams of gold chloride, and dissolve it in an aqueous solution containing 50 g / L of ammonium chloride and 150 g / L of hydrochloric acid at room temperature; Slowly drop 50g / L cysteine at the same temperature (H-Cys stands for cysteine, Cys - Cysteine anion) solution, it is found that turbidity appears in the mixed solution; if necessary, take cooling measures to keep the temperature of the reaction system basically stable, and take airtight measures to prevent air or oxygen from entering the reaction system; continue to drop such Cysteine solution until the precipitate no longer increases; filter the above mixture containing turbidity and wash the resulting precipitate, and store the obtained precipitate in airtight conditions at 0°C-5°C, tentatively named It is an intermediate ①; in addition, mix cysteine and potassium hydroxide at a molar ratio of 1:1 to obtain a potassium cysteine solution, and prepare a solution with a potassium cysteine content...
Embodiment 2
[0034] Take 2.5 grams of gold thiourea {Au (TU) 2}Cl (TU stands for thiourea), dissolved in 100 grams of DI water; slowly drop 50 g / L mercaptoethanol (H- ME stands for mercaptoethanol, H stands for its mercapto active hydrogen H, ME stands for the rest of the solution except H), and it is found that turbid precipitates appear in the mixed solution; if necessary, take cooling measures to maintain the temperature of the reaction system basically stable, and adopt airtight and other methods to block air or oxygen from entering the reaction system; continue to add such H-ME solution dropwise until the precipitate no longer increases; filter the above-mentioned mixture containing the precipitate and wash the resulting precipitate, and the obtained precipitate The product is stored in an anaerobic environment and temporarily named as precipitate ①; in addition, H-ME and potassium hydroxide are mixed according to the molar ratio of 1:1 to obtain a K-ME solution, and the content of K-...
Embodiment 3
[0039] Take 4 grams of potassium chloroaurate, dissolve it in 100 grams of DI water, adjust its pH value to 4.0~6.1 with 50 g / L potassium carbonate solution, and obtain Au (Ⅲ) solution; at a temperature higher than the freezing point of the above mixture solution Add slowly dropwise ammonium thioglycolate (NH 4 + MA - ) Liquid (MA - It represents the corresponding negative monovalent anion that has removed an active hydrogen from the thioglycolic acid molecule), and it is found that a turbid precipitate appears in the mixed solution. If necessary, take cooling measures to maintain the temperature of the reaction system at 5°C, and continue to drop the above mercapto Ammonium acetate until the turbidity no longer increases; the above-mentioned mixture containing the precipitate is filtered and the resulting precipitate is washed, and the obtained precipitate is named intermediate ①; another portion of potassium hydroxide is prepared with a concentration of 0.2M ( mol / L) solu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com