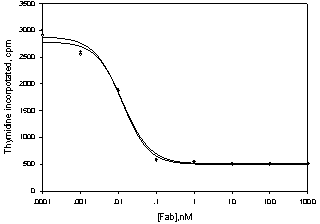
Method for purifying and preparing anti-VEGF antibody fragment
An antibody fragment and purification method technology, which is applied in the field of purification and preparation of anti-VEGF antibody fragments, can solve the problems of easy shedding of ligands, reduced dynamic binding capacity, high price, etc., and achieves simple and convenient operation, improved stability, and simple operation. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] 1. Strain construction and recombinant antibody expression: Construct a humanized anti-vascular endothelial growth factor Fab phage antibody library, use VEGF-A as a ligand for biopanning, obtain highly specific positive clones, compare and compare the sequences after sequencing Analysis; construction of a periplasmic expression vector suitable for the expression of antibody Fab fragments, the vector elements include: E. coli promoter, replicon, antibiotic selection marker, polyclonal restriction site, two ribosome recognition sites, two signal peptides sequence, two terminator , to transcribe and guide the heavy chain and light chain of the antibody respectively, the light chain and the heavy chain guided to the periplasm specifically cut the signal peptide under the action of the enzyme, and the heavy chain and the light chain form a protein whose N-terminus is a natural amino acid sequence, two In the oxidative environment of the periplasmic cavity, the chain forms a...
Embodiment 2
[0074] 1. Strain construction and target protein expression: Construct a humanized anti-vascular endothelial growth factor Fab phage antibody library, use VEGF-A as a ligand for biopanning, obtain highly specific positive clones, compare and compare the sequences after sequencing analyze. Construct a periplasmic expression vector suitable for the expression of antibody Fab fragments. The vector elements include: E. coli promoter, replicon, antibiotic selection marker, polyclonal restriction site, two ribosome recognition sites, two signal peptide sequences, two terminator , to transcribe and guide the heavy chain and light chain of the antibody respectively, the light chain and the heavy chain guided to the periplasm specifically cut the signal peptide under the action of the enzyme, and the heavy chain and the light chain form a protein whose N-terminus is a natural amino acid sequence, two In the oxidative environment of the periplasmic cavity, the chains pair and form disu...
Embodiment 3
[0093] 1. Strain construction and target protein expression: Construct a humanized anti-vascular endothelial growth factor Fab phage antibody library, use VEGF-A as a ligand for biopanning, obtain highly specific positive clones, compare and compare the sequences after sequencing analyze. Construct a periplasmic expression vector suitable for the expression of antibody Fab fragments. The vector elements include: E. coli promoter, replicon, antibiotic selection marker, polyclonal restriction site, two ribosome recognition sites, two signal peptide sequences, Two terminators, which respectively transcribe and guide the heavy chain and light chain of the antibody, guide the light chain and heavy chain to the periplasm to specifically cut the signal peptide under the action of enzymes, and the N-terminus of the heavy chain and light chain is the natural amino acid sequence In the protein, the two chains pair and form disulfide bonds in the oxidative environment of the periplasmic ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com